Neon isotope line split photograph by J. J. Thomson
. Source. J. J. Thomson took this picture in 1912:There can, therefore, I think, be little doubt that what has been called neon is not a simple gas but a mixture of two gases, one of which has an atomic weight about 20 and the other about 22. The parabola due to the heavier gas is always much fainter than that due to the lighter, so that probably the heavier gas forms only a small percentage of the mixture.
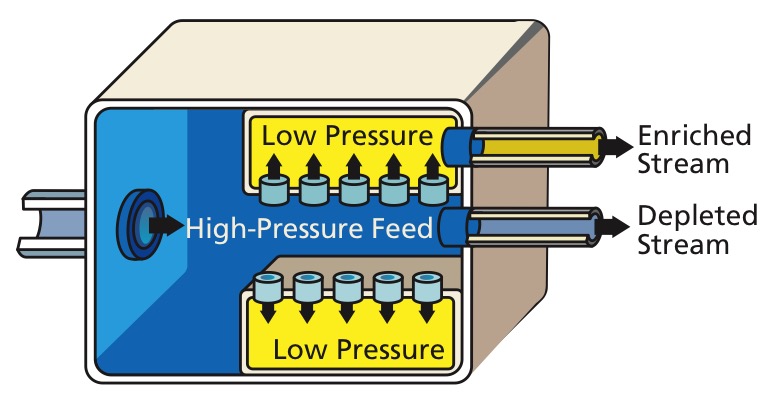
This isotope separation method was the first big successful method, having been used in the Manhattan Project, notably in the K-25 reactor.
This method was superseded by the more efficient gas centrifuges.