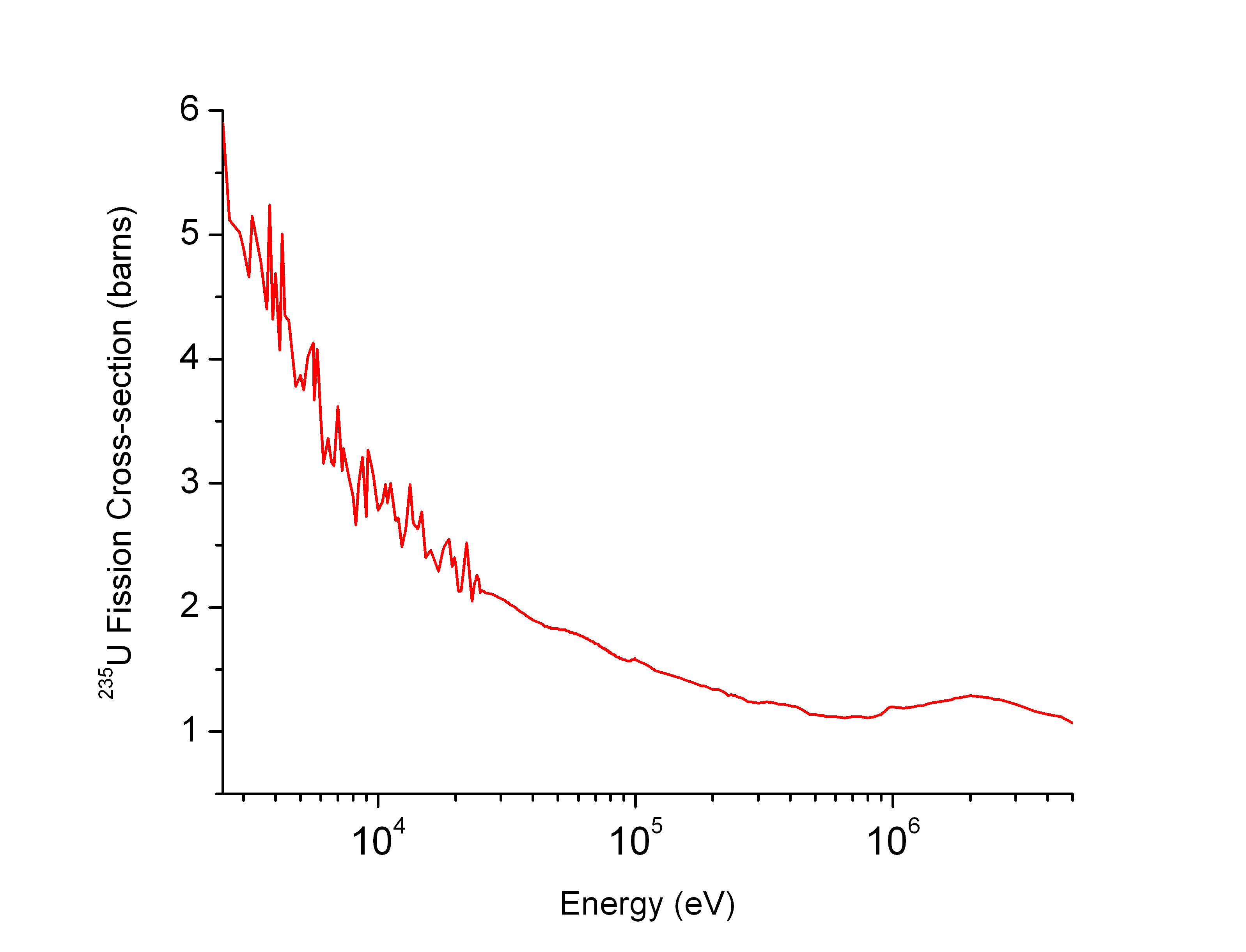
Nuclear physics is basically just the study of the complex outcomes of weak interaction + quantum chromodynamics.
Atomic Physics - An Historical Approach
. Source. By the British Department of Energy. Possibly: www.acmi.net.au/works/114589--atomic-physics-an-historical-approach/ which dates it 1945 - 1947.Side effect of the strong force that in addition to binding individual protons and neutrons as units, also binds different protons and neutrons to one another.
These are neutrons that have reached the thermal equilibrium according to the Maxwell-Boltzmann distribution after having bounced around many times without undergoing neutron capture.
Good fissile material is material that is able to absorb thermal neutrons and continue the reaction, because that's the type of neutron you end up getting the most of.
Some of the most notable ones:
- 1942: Chicago Pile-1: the first human-made nuclear chain reaction.
- 1943: X-10 Graphite Reactor: an intermediate step between the nuclear chain reaction prototype Chicago Pile-1 and the full blown mass production at Hanford site. Located in the Oak Ridge National Laboratory.
- 1944: B Reactor at the Hanford site produced the plutonium used for Trinity and Fat Man
A nuclear reactor made to produce specific isotopes rather than just consume fissile material to produce electrical power. The most notably application being to produce Plutonium-239 for nuclear weapons from Uranium-238 being irradiated from Uranium-235-created fission.
Ciro Santilli finds it interesting that radioactive decay basically kickstarted the domain of nuclear physics by essentially providing a natural particle accelerator from a chunk of radioactive element.
The discovery process was particularly interesting, including Henri Becquerel's luck while observing phosphorescence, and Marie Curie's observation that the uranium ore were more radioactive than pure uranium, and must therefore contain other even more radioactive substances, which lead to the discovery of polonium (half-life 138 days) and radium (half-life 1600 years).
Most of the helium in the Earth's atmosphere comes from alpha decay, since helium is lighter than air and naturally escapes out out of the atmosphere.
Wiki mentions that alpha decay is well modelled as a quantum tunnelling event, see also Geiger-Nuttall law.
As a result of that law, alpha particles have relatively little energy variation around 5 MeV or a speed of about 5% of the speed of light for any element, because the energy is inversely exponentially proportional to half-life. This is because:
Quantum tunnelling and the Alpha particle Paradox by Physics Explained (2022)
Source. - youtu.be/_f8zeEI0oys?t=796 George Gamow and Edward Condon proposed the quantum tunnelling explanation
- youtu.be/_f8zeEI0oys?t=1725 worked out example that predicts the half-life of polonium-210 based on its emission energy
They are stopped by:Therefore, alpha emitters are not too dangerous unless ingested.
Uranium emits them, you can see their mass to charge ratio under magnetic field and so deduce that they are electrons.
Caused by weak interaction TODO why/how.
The emitted electron kinetic energy is random from zero to a maximum value. The rest goes into a neutrino. This is how the neutrino was first discovered/observed indirectly. This is well illustrated in a decay scheme such as Figure "caesium-137 decay scheme".
Their energy is very high compared example to more common radiation such as visible spectrum, and there is a neat reason for that: it's because the strong force that binds nuclei is strong so transitions lead to large energy changes.
A decay scheme such as Figure "caesium-137 decay scheme" illustrates well how gamma radiation happens as a byproduct of radioactive decay due to the existence of nuclear isomer.
Gamma rays are pretty cool as they give us insight into the energy levels/different configurations of the nucleus.
They have also been used as early sources of high energy particles for particle physics experiments before the development of particle accelerators, serving a similar purpose to cosmic rays in those early days.
But gamma rays they were more convenient in some cases because you could more easily manage them inside a laboratory rather than have to go climb some bloody mountain or a balloon.
The positron for example was first observed on cosmic rays, but better confirmed in gamma ray experiments by Carl David Anderson.
Gamma spectroscopy of a Uranium ore
. Source. Several points of the Uranium 238 decay chain are clearly visible.The following cobalt-60 diagrams suggest that some lines are clearly visible in specific nuclear reactions:
Also this one:
Example: Figure "caesium-137 decay scheme"
The half-life of radioactive decay, which as discovered a few years before quantum mechanics was discovered and matured, was a major mystery. Why do some nuclei fission in apparently random fashion, while others don't? How is the state of different nuclei different from one another? This is mentioned in Inward Bound by Abraham Pais (1988) Chapter 6.e Why a half-life?
The term also sees use in other areas, notably biology, where e.g. RNAs spontaneously decay as part of the cell's control system, see e.g. mentions in E. Coli Whole Cell Model by Covert Lab.
Neon isotope line split photograph by J. J. Thomson
. Source. J. J. Thomson took this picture in 1912:There can, therefore, I think, be little doubt that what has been called neon is not a simple gas but a mixture of two gases, one of which has an atomic weight about 20 and the other about 22. The parabola due to the heavier gas is always much fainter than that due to the lighter, so that probably the heavier gas forms only a small percentage of the mixture.
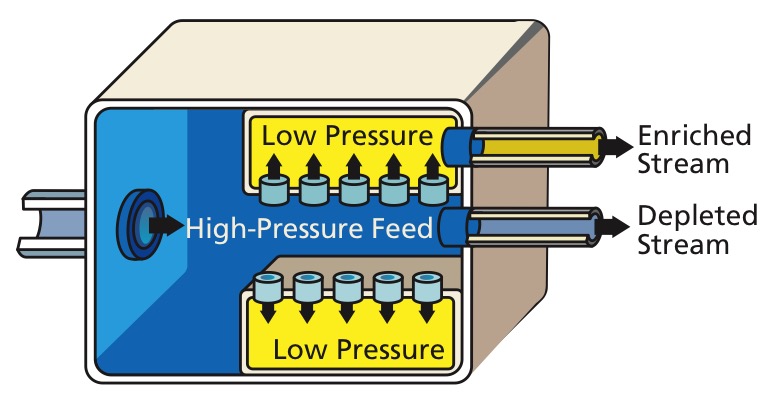
This isotope separation method was the first big successful method, having been used in the Manhattan Project, notably in the K-25 reactor.
This method was superseded by the more efficient gas centrifuges.
Ciro Santilli once visited the chemistry department of a world leading university, and the chemists there were obsessed with NMR. They had small benchtop NMR machines. They had larger machines. They had a room full of huge machines. They had them in corridors and on desk tops. Chemists really love that stuff. More precisely, these are used for NMR spectroscopy, which helps identify what a sample is made of.
Introduction to NMR by Allery Chemistry
. Source. - only works with an odd number of nucleons
- apply strong magnetic field, this separates the energy of up and down spins. Most spins align with field.
- send radio waves into sample to make nucleons go to upper energy level. We can see that the energy difference is small since we are talking about radio waves, low frequency.
- when nucleon goes back down, it re-emits radio waves, and we detect that. TODO: how do we not get that confused with the input wave, which is presumably at the same frequency? It appears to send pulses, and then wait for the response.
How to Prepare and Run a NMR Sample by University of Bath (2017)
Source. This is a more direct howto, cool to see. Uses a Bruker Corporation 300. They have a robotic arm add-on. Shows spectrum on computer screen at the end. Shame no molecule identification after that!This video has the merit of showing real equipment usage, including sample preparation.
Says clearly that NMR is the most important way to identify organic compounds.
- youtu.be/uNM801B9Y84?t=41 lists some of the most common targets, including hydrogen and carbon-13
- youtu.be/uNM801B9Y84?t=124 ethanol example
- youtu.be/uNM801B9Y84?t=251 they use solvents where all protium is replaced by deuterium to not affect results. Genius.
- youtu.be/uNM801B9Y84?t=354 usually they do 16 radio wave pulses
Introductory NMR & MRI: Video 01 by Magritek (2009)
Source. Precession and Resonance. Precession has a natural frequency for any angle of the wheel.Introductory NMR & MRI: Video 02 by Magritek (2009)
Source. The influence of temperature on spin statistics. At 300K, the number of up and down spins are very similar. As you reduce temperature, we get more and more on lower energy state.Introductory NMR & MRI: Video 03 by Magritek (2009)
Source. The influence of temperature on spin statistics. At 300K, the number of up and down spins are very similar. As you reduce temperature, we get more and more on lower energy state.NMR spectroscopy visualized by ScienceSketch
. Source. 2020. Decent explanation with animation. Could go into a bit more numbers, but OK.Bibliography:
Used to identify organic compounds.
Seems to be based on the effects that electrons around the nuclei (shielding electrons) have on the outcome of NMR.
So it is a bit unlike MRI where you are interested in the position of certain nuclei in space (of course, these being atoms, you can't see their positions in space).
What's Nuclear Magnetic Resonance by Bruker Corporation (2020)
Source. Good 3D animations showing the structure of the NMR machine. We understand that it is very bulky largely due to the cryogenic system. It then talks a bit about organic compound identification by talking about ethanol, i.e. this is NMR spectroscopy, but it is a bit too much to follow closely. Basically the electron configuration alters the nuclear response somehow, and allows identifying functional groups.How MRI Works Part 1 by thePIRL (2018)
Source. - youtu.be/TQegSF4ZiIQ?t=326 the magnet is normally always on for the entire lifetime of the equipment!
- youtu.be/TQegSF4ZiIQ?t=465 usage of non-ionizing radiation (only radio frequencies) means that it is very safe to use. The only dangerous part is the magnetic field interacting with metallic objects.
Dr Mansfield's MRI MEDICAL MARVEL by BBC
. Source. Broadcast in 1978. Description:Tomorrow's World gave audiences a true world first as Dr Peter Mansfield of the University of Nottingham demonstrated the first full body prototype device for Magnetic resonance imaging (MRI), allowing us to see inside the human body without the use of X-rays.
A weapons-grade ring of electrorefined plutonium, typical of the rings refined at Los Alamos and sent to Rocky Flats for fabrication
. Source. The ring has a purity of 99.96%, weighs 5.3 kg, and is approx 11 cm in diameter. It is enough plutonium for one bomb core. Which city shall we blow up today?Ciro Santilli is mildly obsessed by nuclear reactions, because they are so quirky. How can a little ball destroy a city? How can putting too much of it together produce criticality and kill people like in the Slotin accident or the Tokaimura criticality accident. It is mind blowing really.
More fun nuclear stuff to watch:
- Dr. Strangelove (1964)
- en.wikipedia.org/wiki/Chernobyl_(miniseries)
- The World Of Enrico Fermi by Harvard Project Physics (1970)
- Fat Man and Little Boy (1987) shows a possibly reasonably realistic of the history of the development of the Trinity
The Ultimate Guide to Nuclear Weapons by hypohystericalhistory (2022)
Source. Good overall summary. Some interesting points:- youtu.be/8uIPQBOCJ64?t=2946 talks about the difference between tactical and strategic nuclear weapons
- youtu.be/8uIPQBOCJ64?t=3291 mentions variable yield devices, this is the main new thing Ciro Santilli learned from this video
- youtu.be/8uIPQBOCJ64?t=3416 discusses if a strategic nuclear weapon usage would inevitably lead to tactical nuclear weapon escalation. It then mentions one case in which a possibly comparable escalation didn't happen: the abstinence of using chemical weapon during World War II.
Gun-type fission weapons are the simplest approach and they work with Uranium-235 bombs as you can ignite it with just one explosion.
But Gun-type fission weapons don't work with plutonium, and weapon grade Plutonium is cheaper than weapon grade Uranium, so it wasn't much used.
Gun-type fission weapons don't work with Plutonium-239 because of the presence of Plutonium-240 as an impurity which leads to fizzle.
Good mentions at: youtu.be/dgBDvnqMkT4?t=252
Implosion-type fission weapons are more complicated than gun-type fission weapon because you have to precisely coordinate the detonation of a bunch of explosives.
Oppenheimer's Gamble by Welch Labs
. Source. Fails to mention Plutonium-240 as the source of the problem.- youtu.be/W06g7gIfwRE?t=252 cool to learn that the explosive lenses had to be made out of two different types of explosives, slow and fast, to be more symmetrical
Production is fully concentrated at Los Alamos National Laboratory in the United States as of 2020. TODO was it ever made anywhere else?
For nuclear weapons you need a certain level of isotope purity of either plutonium-239 or uranium-235.
And the easiest way by far to achieve this purity is to produce plutonium-239 in a breeder reactor, which allows you to get it out with much cheaper chemical processes rather than costly isotope separation methods.
sgp.fas.org/othergov/doe/pu50yc.html mentions:That site also contains a good summary of the closed shutdown reactors in each site. These are publicly disclosed e.g. at: www.hanford.gov/page.cfm/ProjectsFacilities
The United States Government has used 14 plutonium production reactors at the Hanford and Savannah River sites to produce plutonium for the U.S. nuclear weapons stockpile and DOE research and development programs. From 1944 to 1994, these reactors produced 103.4 metric tons of plutonium; 67.4 MT at Hanford, and 36.1 at Savannah River.
www.youtube.com/watch?v=qwKhz7BPBLY mentions that before they stopped production at the end of the Cold War, Hanford site produced 2/3 of the total American stockpile, and Savannah River site produced 1/3.
www.reuters.com/article/world/americas-nuclear-headache-old-plutonium-with-nowhere-to-go-idUSKBN1HR1JT/ claims that as of 2018 Savannah River site stored the majority of the stockpile.
Bibliography:
nct-cbnw.com/americas-new-nukes-plutonium-pits-at-los-alamos/
After the closure of the country’s prime plutonium manufacturing plant at Rocky Flats in 1992, where 1,000 to 2,000 pits were produced every year, a highly reinforced 236,000 sq-ft facility built at LANL earlier in 1978 became the first Department of Energy (DoE) facility capable of producing plutonium cores.Although initially established for plutonium research and development, in 2003 the Plutonium Facility Building 4 (PF-4) within Tech Area 55 at Los Alamos produced the nation’s stockpile quality (first war reserve) plutonium. In 2006, Congress instructed the DoE to focus on producing pits at this facility.
The B Reactor of the facility produced the plutonium used for Trinity and Fat Man, and then for many more thousand bombs during the Cold War. More precisely, this was done at
Located in Washington, in a dry place the middle of the mountainous areas of the Western United States, where basically no one lives. The Columbia river is however nearby, that river is quite large, and provided the water needed by their activities, notably for cooling the nuclear reactors. It is worth it having look on Google Maps to get a feel for the region.
Unlike many other such laboratories, this one did not become a United States Department of Energy national laboratories. It was likely just too polluted.
Bibliography:
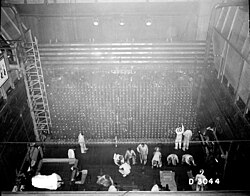
This was the first full scale nuclear reactor in the world, and was brought up slowly to test it out.
Hanford B Reactor tour by Studio McGraw
. Source. 2016.- youtu.be/8rlVHEY7BF0?t=335 good description of the fuel element. It uses uranium metal, not Uranium dioxide
- youtu.be/8rlVHEY7BF0?t=652 N Reactor and F Reactor were identical, and came up 2 months later, but much faster because of what they learned on the B
This was one of the two American plutonium production sites, together with the Hanford site. www.youtube.com/watch?v=qwKhz7BPBLY mentions it produced about 1/3 of the total American plutonium.
www.reuters.com/article/world/americas-nuclear-headache-old-plutonium-with-nowhere-to-go-idUSKBN1HR1JT/ claims that as of 2018 it stored the majority of the plutonium stockpile of the country.
It's where most American nuclear weapons are assembled and disassembled.
Dismantlement of Nuclear Weapons by Sandia National Laboratories
. Source. Great video, shows everything that is not classified.Historic, unique Manhattan Project footage from Los Alamos by Los Alamos National Lab
. Source. Mostly the daily life part of things, but very good, includes subtitles explaining the people and places shown.
Marked with identifier "LA-UR 11-4449".
Getting funding for the Chicago Pile Edward Teller interview by Web of Stories (1996)
Source. - youtu.be/mnScq24BEmc?t=114 the main cost for the reactor was the graphite. Presumably they already had the uranium in hand?. Edit, no, it is because it was a specialized graphite: Video 2. "German graphite from The Genius Behind the Bomb (1992)", i.e. nuclear graphite.
German graphite from The Genius Behind the Bomb (1992)
Source. Graphite was expensive because it had to be boron-free, since boron absorbs neutrons. But a boron process was the main way to make graphite. This type of pure graphite is known as nuclear graphite.The lab that made Chicago Pile-1, located in the University of Chicago. Metallurgical in this context basically as in "working with the metals uranium and plutonium".
Given their experience, they also designed the important X-10 Graphite Reactor and the B Reactor which were built in other locations.
Plutonium-based.
Its plutonium was produced at Hanford site.
Trinity: Getting The Job Done
. Source. Good video, clarifies several interesting technical points:- Gun-type fission weapon were much easier to build as you don't need super synchronized charges as in implosion-type fission weapon. But they are less efficient.
- Plutonium make much more efficient usage of uranium, because you don't need to highly enrich a bunch of Uranium-235 in the first place, but rather just use way less enriched Uranium-235 to produce a bunch of Plutonium by converting Uranium-238
Their website, and in particular the recruitment section, are so creepy.
There's not mention of bombs. No photos of atomic explosions. The words "atomic" and "weapon" do not even show up in the front page!!! The acronym AWE is instead used everywhere as an euphemism.
In the recruitment section we can see a bunch of people smiling: web.archive.org/web/20211007213222/https://www.awe.co.uk/careers/working-at-awe/, suggesting:There's even children outreach!!!
Ciro Santilli is not against storing a few nukes to be ready against dictatorships. But don't be such a pussy! Just say what the fuck you are doing more clearly! You are making weapons to kill people and destroy things in order to maintain the Balance of power. If the public can't handle such facts, then shut down the fucking program.
Atomic Weapons Establishment by The Gwent Auditor
. Source. Dude walking around the public area filming. Got stopped by police to make sure he didn't do anything he shouldn't.Knock knock.
Missileers by BBC (2000)
Source. Documentary about American ICBM crews working on the Francis. E. Warren Air Force Base. Wiki mentions that there are 3 main sites in the USA, and plainshumanities.unl.edu/encyclopedia/doc/egp.ii.042 suggests all/most of them are in the Great Plains area. They operate a Minuteman system, which as of 2021 is the only nuclear ICBM system in the USA.
Good documentary, shows well the day-to-day life of the operator, including outside of the work site.
- youtu.be/w1tMx27Q4O0?t=1390 they drive 100 miles to get to work. They do 8 alerts per month.
- youtu.be/w1tMx27Q4O0?t=1473 the actual missiles are a few miles away from the control center, scattered in a few different locations
- youtu.be/w1tMx27Q4O0?t=1619 they have a television in there at least. Presumably a pre-recorded selection.
Logistics support management by USAF
. Source. Shows logistic operations behind the American ICBM system of the time. Reuploaded to showcase the IBM 705 system used to track parts, notably the usage of a punch cards.alcpress.org/military/icbm/index.html has a Google Maps overlay with all of the American ICBM sites, spread out across three centers. It is cute to see how they are very evenly spread out to make it hard to take them out at once.
uploads.fas.org/sites/4/NotebookMap.pdf summarizes all ICBM and also other delivery methods as of 2006.
Ah, the choice of name, both grim and slightly funny, Dr. Strangelove comes to mind quite strongly. Also Fallout (franchise).
- youtu.be/VTQ8yZSyrC0?t=75 map of missile silos
- youtu.be/VTQ8yZSyrC0?t=210 shows a map of the communication copper wires linking up a silo farm. Presumably Hardened Intersite Cable System
Bibliography:
Ciro Santilli's jaw dropped when he learned about this concept. A Small Talent for War, are you sure?
Articles were limited to the first 100 out of 107 total. Click here to view all children of Nuclear physics.
Articles by others on the same topic
Nuclear physics is the branch of physics that deals with the study of atomic nuclei, their constituents (protons and neutrons), and the interactions that occur between them. It encompasses a variety of topics, including: 1. **Structure of the Nucleus**: Understanding the arrangement of protons and neutrons within an atomic nucleus, including models that describe nuclear stability and the forces that hold the nucleus together (strong nuclear force).