Joseph Priestley in 1801
. Source. Welcome to my home page!
Cryptocurrency Romance Scam Recovery Expert - Fast Swift Cyber Services Today by  Gary Howard 0 Created 2024-12-31 Updated 2025-04-18
Gary Howard 0 Created 2024-12-31 Updated 2025-04-18
Cryptocurrency Romance Scam Recovery Expert - Fast Swift Cyber Services Today
If you've been a victim of a romance cryptocurrency scam, you understand the pain and frustration of losing your money to fraudsters who prey on trust. Fast Swift Cyber Services are specialist who has earned a reputation for helping individuals recover funds lost in these scams. I had the unfortunate experience of being scammed by a fraudulent cryptocurrency investment scheme, and after months of feeling helpless, I reached out to Fast Swift Cyber Services for assistance.
If you've been a victim of a romance cryptocurrency scam, you understand the pain and frustration of losing your money to fraudsters who prey on trust. Fast Swift Cyber Services are specialist who has earned a reputation for helping individuals recover funds lost in these scams. I had the unfortunate experience of being scammed by a fraudulent cryptocurrency investment scheme, and after months of feeling helpless, I reached out to Fast Swift Cyber Services for assistance.
Fast Swift Cyber Services kept me updated regularly, answering any questions I had and offering advice on how to avoid similar scams in the future. What impressed me most was their commitment and their ethical approach. They never made unrealistic promises or guarantees, but they worked tirelessly on my case, and after some time, I saw significant progress in recovering part of my lost funds.
If you find yourself in a similar situation, I highly recommend Fast Swift Cyber Services.
FAST SWIFT CYBER SERVICES:
FAST SWIFT CYBER SERVICES:
WhatsApp : +1 419,763.9173
fastswift@cyberservices . com
fastswift@cyberservices . com
Welcome to my home page!
HOW TO RECOVER FROM FRAUDULENT CRYPTO INVESTMENT PLATFORM → CONTACT HACKATHON TECH SOLUTIONS by  Richard Walton 0 Created 2024-12-30 Updated 2025-04-18
Richard Walton 0 Created 2024-12-30 Updated 2025-04-18
Are you looking for a professional Bitcoin/USDT/ETH recovery expert to help you recover your lost or stolen cryptocurrency? Look no further than Hackathon Tech Solutions. Our team of experts has years of experience in recovering lost or stolen cryptocurrency and can help you get back your funds quickly and securely. Don't let your hard-earned money go to waste, contact us today for a free consultation and let us help you recover your cryptocurrency. Reach out to HACKATHON TECH SOLUTIONS via below contact details
Email: info@hackathontechsolution.com
Telegram: @hackathontechsolutions
Welcome to my home page!
Welcome to my home page!
Lyrics: 1983 by Wang Jiazhen.
Music: 1995 by Hu Xiaohuan.
Lyrics:
门前大桥下
In front of the door, under the bridge快来快来数一数
Quickly come count them二四六七八
Two, four, six, seven, eight咕嘎咕嘎
Quack, quack, quack赶鸭老爷爷
Old man herding ducks (The documentary Most Dangerous Ways To School - Vietnam for example shows a fantastic example of a person herding ducks on a river in Vietnam)胡子白花花
with a shinning white beard唱呀唱着家乡戏
He sings his hometown opera (TODO this deserves further invetigation and examples)还会说笑话
and he can also tell jokes小孩,小孩
Children, children快快上学校
quickly go to school
Let's create some test data like this:
time psql tmp -c 'DROP TABLE IF EXISTS fts;'
time psql tmp -c 'CREATE TABLE fts(s TEXT, i INTEGER);'
time psql tmp <<'EOF'
INSERT INTO fts SELECT
i::text || ' ' ||
(i * 2 )::text || ' ' ||
(i * 5 )::text || ' ' ||
(i * 7 )::text || ' ' ||
(i * 11 )::text || ' ' ||
(i * 13 )::text || ' ' ||
(i * 17 )::text || ' ' ||
(i * 23 )::text || ' ' ||
(i * 29 )::text || ' ' ||
(i * 31 )::text
,
i % 100
FROM generate_series(1::bigint, 100000000::bigint) AS s(i);
EOFThe creation time was 2m13s, and the final size was:
table_name | pg_size_pretty | pg_total_relation_size
------------------+----------------+------------------------
fts | 13 GB | 14067326976This test data will be simple to predict what each line contains so we can make educated queries, while also posing some difficulty to the RDMS. As per:the first columns look like:
time psql tmp -c 'SELECT * FROM fts LIMIT 10;' s | i
-------------------------------------+----
1 2 5 7 11 13 17 23 29 31 | 1
2 4 10 14 22 26 34 46 58 62 | 2
3 6 15 21 33 39 51 69 87 93 | 3
4 8 20 28 44 52 68 92 116 124 | 4
5 10 25 35 55 65 85 115 145 155 | 5
6 12 30 42 66 78 102 138 174 186 | 6
7 14 35 49 77 91 119 161 203 217 | 7
8 16 40 56 88 104 136 184 232 248 | 8
9 18 45 63 99 117 153 207 261 279 | 9
10 20 50 70 110 130 170 230 290 310 | 10We aimed to create a test table of size around 10 GB, as in practice it is around that order of size that index speedups start to become very obvious on a SSD-based system.
Before we create the index, let's see if our non-indexed queries are slow enough for our tests:which gives:so it should be enough to observe the index speedup.
time psql tmp -c "SELECT * FROM fts WHERE s LIKE '% 50000000 %';" s | i
---------------------------------------------------------------------------------------------------+---
10000000 20000000 50000000 70000000 110000000 130000000 170000000 230000000 290000000 310000000 | 0
25000000 50000000 125000000 175000000 275000000 325000000 425000000 575000000 725000000 775000000 | 0
(2 rows)
real 0m11.758s
user 0m0.017s
sys 0m0.008sNow let's create the index. First we create a generated column that splits the strings with These commands took 8m51s and 40m8s and the DB size went up about 5x:
to_tsvector, and then we index that split column:time psql tmp <<'EOF'
ALTER TABLE fts ADD COLUMN s_ts tsvector
GENERATED ALWAYS AS (to_tsvector('english', s)) STORED;
EOF
time psql tmp -c 'CREATE INDEX s_ts_gin_idx ON fts USING GIN (s_ts);' table_name | pg_size_pretty | pg_total_relation_size
------------------+----------------+------------------------
fts | 69 GB | 74487758848And finally let's try out the index:which "instantly" gives us in 0m0.129s:so the index worked!
time psql tmp -c "SELECT s, i FROM fts WHERE s_ts @@ to_tsquery('english', '50000000');" s | i
-------------------------------------------------------------------------------------------------------+---
10000000 20000000 50000000 70000000 110000000 130000000 170000000 230000000 290000000 310000000 | 0
25000000 50000000 125000000 175000000 275000000 325000000 425000000 575000000 725000000 775000000 | 0
50000000 100000000 250000000 350000000 550000000 650000000 850000000 1150000000 1450000000 1550000000 | 0Another important use case is to search for prefixes of words, e.g. as you'd want in a simple autocompletion system. This can be achieved by adding This finishes in the same amount of time, and gives:so now we have cool hits such as
:* at the end of the search term as in:time psql tmp -c "SELECT s, i FROM fts WHERE s_ts @@ to_tsquery('english', '50000000:*');" s | i
-----------------------------------------------------------------------------------------------------------+----
10000000 20000000 50000000 70000000 110000000 130000000 170000000 230000000 290000000 310000000 | 0
38461539 76923078 192307695 269230773 423076929 500000007 653846163 884615397 1115384631 1192307709 | 39
45454546 90909092 227272730 318181822 500000006 590909098 772727282 1045454558 1318181834 1409090926 | 46
50000000 100000000 250000000 350000000 550000000 650000000 850000000 1150000000 1450000000 1550000000 | 0
71428572 142857144 357142860 500000004 785714292 928571436 1214285724 1642857156 2071428588 2214285732 | 72
100000000 200000000 500000000 700000000 1100000000 1300000000 1700000000 2300000000 2900000000 3100000000 | 0
29411765 58823530 147058825 205882355 323529415 382352945 500000005 676470595 852941185 911764715 | 65
25000000 50000000 125000000 175000000 275000000 325000000 425000000 575000000 725000000 775000000 | 0500000000, 500000004, 500000005, 500000007 and 500000006. The syntax is also mentioned at:Next we can also try some other queries with multiple terms. Text must contain two words with gives:
&:time psql tmp -c "SELECT s, i FROM fts WHERE s_ts @@ to_tsquery('english', '50000000 & 175000000');" s | i
-------------------------------------------------------------------------------------------------------+---
25000000 50000000 125000000 175000000 275000000 325000000 425000000 575000000 725000000 775000000 | 0Text can contain either word with gives:
|:time psql tmp -c "SELECT s, i FROM fts WHERE s_ts @@ to_tsquery('english', '50000000 | 175000000');" s | i
---------------------------------------------------------------------------------------------------------+---
10000000 20000000 50000000 70000000 110000000 130000000 170000000 230000000 290000000 310000000 | 0
50000000 100000000 250000000 350000000 550000000 650000000 850000000 1150000000 1450000000 1550000000 | 0
87500000 175000000 437500000 612500000 962500000 1137500000 1487500000 2012500000 2537500000 2712500000 | 0
25000000 50000000 125000000 175000000 275000000 325000000 425000000 575000000 725000000 775000000 | 0
35000000 70000000 175000000 245000000 385000000 455000000 595000000 805000000 1015000000 1085000000 | 0Text can contain the given words sequentially:gives:
time psql tmp -c "SELECT s, i FROM fts WHERE s_ts @@ to_tsquery('english', '50000000 <-> 125000000 <-> 175000000');" s | i
-------------------------------------------------------------------------------------------------------+---
25000000 50000000 125000000 175000000 275000000 325000000 425000000 575000000 725000000 775000000 | 0We can also inspect how words were split by simply doing a
SELECT * again: s | i | s_ts
----------------------------+---+----------------------------------------------------------------------
1 2 5 7 11 13 17 23 29 31 | 1 | '1':1 '11':5 '13':6 '17':7 '2':2 '23':8 '29':9 '31':10 '5':3 '7':4
2 4 10 14 22 26 34 46 58 62 | 2 | '10':3 '14':4 '2':1 '22':5 '26':6 '34':7 '4':2 '46':8 '58':9 '62':10
3 6 15 21 33 39 51 69 87 93 | 3 | '15':3 '21':4 '3':1 '33':5 '39':6 '51':7 '6':2 '69':8 '87':9 '93':10Let's check if the index updates automatically when we do an insert and if insertion seems to have been significantly slowed down by the index:finishes in:so performance is OK. Presumably, the insertion time is proportional to the number of tokens, doing one logarithmic operation per token, so indexing short chunks of text like titles is easy. And then let's find it:which finds it with:so we are all good. Unfortunately, accurate performance benchmarking is a bit harder than that, as the index by default first collects a certain number of updates into memory into the "pending list", before actually inserting them all at once after a certain mass is reached, as documented at: www.postgresql.org/docs/17/gin.html#GIN-IMPLEMENTATION. We are not going that deep today.
time psql tmp -c "INSERT INTO fts VALUES ('abcd efgh', 99)"real 0m0.043s
user 0m0.014s
sys 0m0.010stime psql tmp -c "SELECT s, i FROM fts WHERE s_ts @@ to_tsquery('english', 'efgh');" s | i
-----------+----
abcd efgh | 99The next thing that we need to understand is how gives:so we understand some of the heuristic normalizations:
to_tsvector tokenizes strings for the english language. For example running:psql -c "select to_tsvector('english', 'A Dog run runs fast faster two Cats: b c to from 1 é befhyph-afthyph.')"'1':13
'afthyph':17
'b':9
'befhyph':16
'befhyph-afthyph':15
'c':10
'cat':8
'dog':2
'fast':5
'faster':6
'run':3,4
'two':7
'é':14- prepositions like
toandfromare gone. These are called stopwords as documented at: www.postgresql.org/docs/17/textsearch-controls.html#TEXTSEARCH-PARSING-DOCUMENTS - words are lowercased and singularized, e.g.
Catsbecomescat - hyphenated words are stored both in separate components and in the full hyphenated form:
'afthyph':17'befhyph':16'befhyph-afthyph':15
The full list of languages available can be obtained with:On Ubuntu 24.10, the list contains major world languages, plus the special gives:so we understand that it is similar to
psql -c '\dF'simple configuration such that:psql -c "select to_tsvector('simple', 'A Dog run runs fast faster two Cats: b c to from 1 é befhyph-afthyph.')"'1':13
'a':1
'afthyph':17
'b':9
'befhyph':16
'befhyph-afthyph':15
'c':10
'cats':8
'dog':2
'fast':5
'faster':6
'from':12
'run':3
'runs':4
'to':11
'two':7
'é':14english but it does not:- seem to have any stopwords
- do singularization normalization
From the query side of things, if the query is going to be open to end users on a web interface, we need to understand giving:To avoid such errors, we can use:Bibliography:
to_tsquery better. The issue is that to_tsquery is quite brutal and happily throws errors for common things users might do e.g. spaces:select to_tsquery('english', 'abc def');ERROR: syntax error in tsquery: "abc def"plainto_tsquery: ANDs everythingwebsearch_to_tsquery: supportsANDwith spaces, OR withor, word negation with-wordand concatenation with"my word". But it unfortunately does not support prefixing, which is what everyone and their mother wants for autocomplete: stackoverflow.com/questions/14103880/escaping-special-characters-in-to-tsquery#comment78452351_41804957
- stackoverflow.com/questions/16020164/psqlexception-error-syntax-error-in-tsquery/16020565#16020565
- stackoverflow.com/questions/14103880/escaping-special-characters-in-to-tsquery
- www.reddit.com/r/rails/comments/1cmloa6/sanitizing_a_search_phrase_when_using_to_tsvector/
- stackoverflow.com/questions/6735881/to-tsquery-validation
- dba.stackexchange.com/questions/135030/filtering-special-characters-in-to-tsquery
Also posted at:
- www.reddit.com/r/PostgreSQL/comments/12yld1o/comment/m3l5nkv/ "Is it worth using Postgres' builtin full-text search or should I go straight to Elastic?", high top Google result for "PostgreSQL full text search" as of 2024. Random, but it's there.
This is how Marie Curie was able to precisely detect radioactivity, which then led to her discovery of new elements.
Another Australian company and using a similar approach as Silicon Quantum Computing:Some coverage at: www.afr.com/technology/start-up-says-it-will-have-a-quantum-computer-by-2028-20240219-p5f64k
Through the company Silicon Quantum Computing, this has been Australia's national quantum computing focus.
This can be used to detect the ionization of air by radiation , see e.g. youtu.be/CZ7DoLLwW04?t=76 from Video "Ions produced by radiation carry a current by Institute of Physics".
sqc.com.au/2024/02/08/silicon-quantum-computing-demonstrates-high-fidelity-initialisation-of-nuclear-spins-in-a-4-qubit-device/ points to one of their papers: www.nature.com/articles/s41565-023-01596-9 High-fidelity initialization and control of electron and nuclear spins in a four-qubit register
Their approach seems to be more precisely called: Kane quantum computer and uses phosphorus embedded in silicon.
They come from the University of New South Wales.
Pinned article: Introduction to the OurBigBook Project
Welcome to the OurBigBook Project! Our goal is to create the perfect publishing platform for STEM subjects, and get university-level students to write the best free STEM tutorials ever.
Everyone is welcome to create an account and play with the site: ourbigbook.com/go/register. We belive that students themselves can write amazing tutorials, but teachers are welcome too. You can write about anything you want, it doesn't have to be STEM or even educational. Silly test content is very welcome and you won't be penalized in any way. Just keep it legal!
Intro to OurBigBook
. Source. We have two killer features:
- topics: topics group articles by different users with the same title, e.g. here is the topic for the "Fundamental Theorem of Calculus" ourbigbook.com/go/topic/fundamental-theorem-of-calculusArticles of different users are sorted by upvote within each article page. This feature is a bit like:
- a Wikipedia where each user can have their own version of each article
- a Q&A website like Stack Overflow, where multiple people can give their views on a given topic, and the best ones are sorted by upvote. Except you don't need to wait for someone to ask first, and any topic goes, no matter how narrow or broad
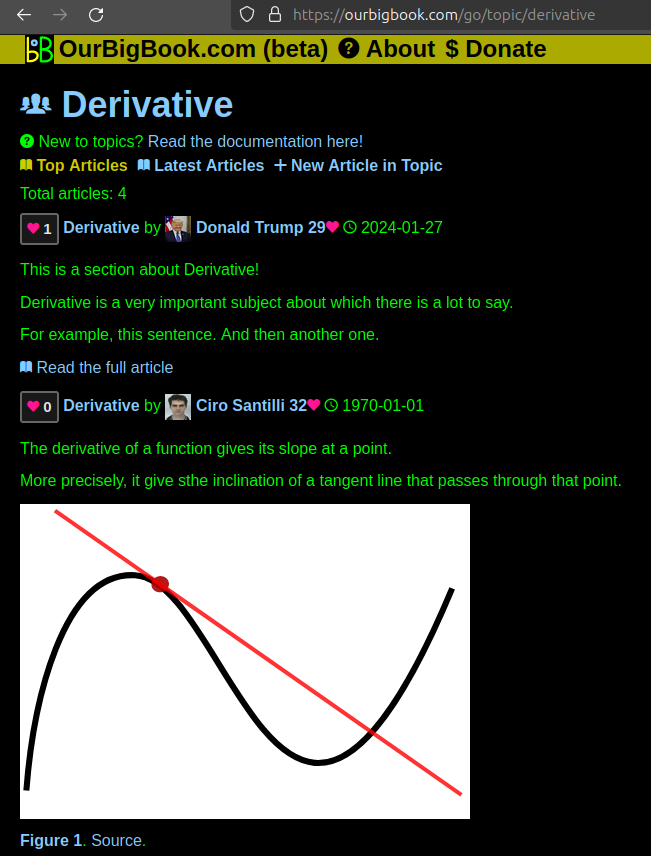
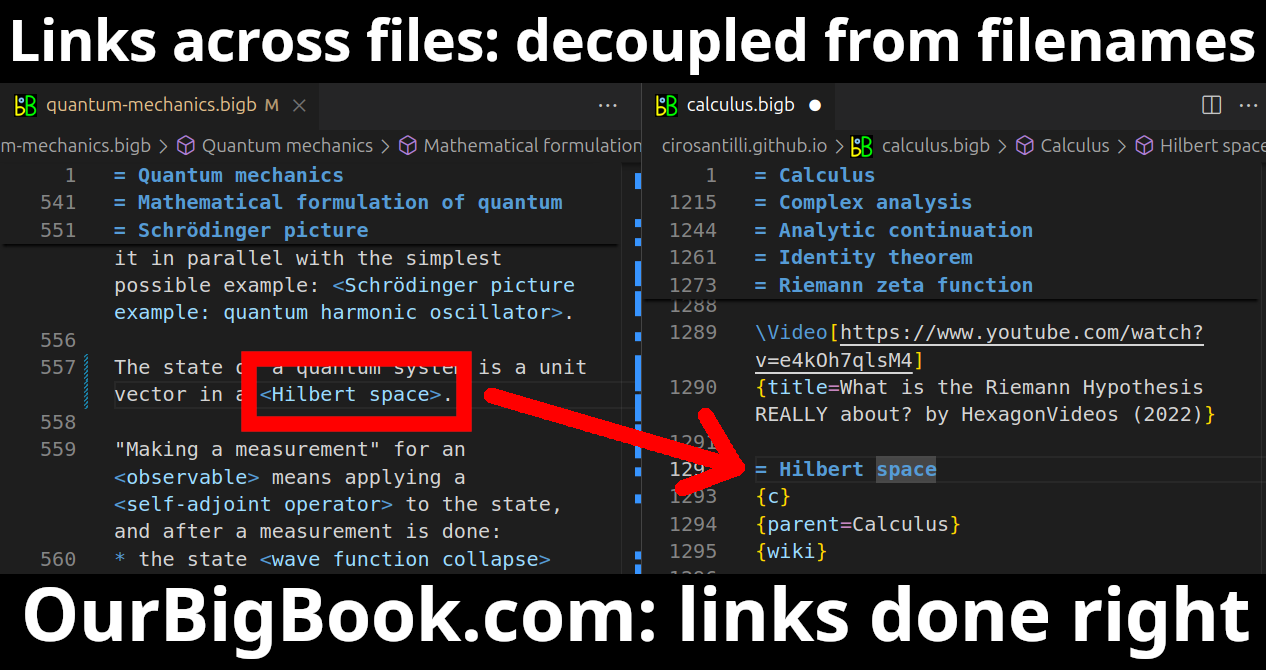
This feature makes it possible for readers to find better explanations of any topic created by other writers. And it allows writers to create an explanation in a place that readers might actually find it.Figure 1. Screenshot of the "Derivative" topic page. View it live at: ourbigbook.com/go/topic/derivativeVideo 2. OurBigBook Web topics demo. Source. - local editing: you can store all your personal knowledge base content locally in a plaintext markup format that can be edited locally and published either:This way you can be sure that even if OurBigBook.com were to go down one day (which we have no plans to do as it is quite cheap to host!), your content will still be perfectly readable as a static site.
- to OurBigBook.com to get awesome multi-user features like topics and likes
- as HTML files to a static website, which you can host yourself for free on many external providers like GitHub Pages, and remain in full control
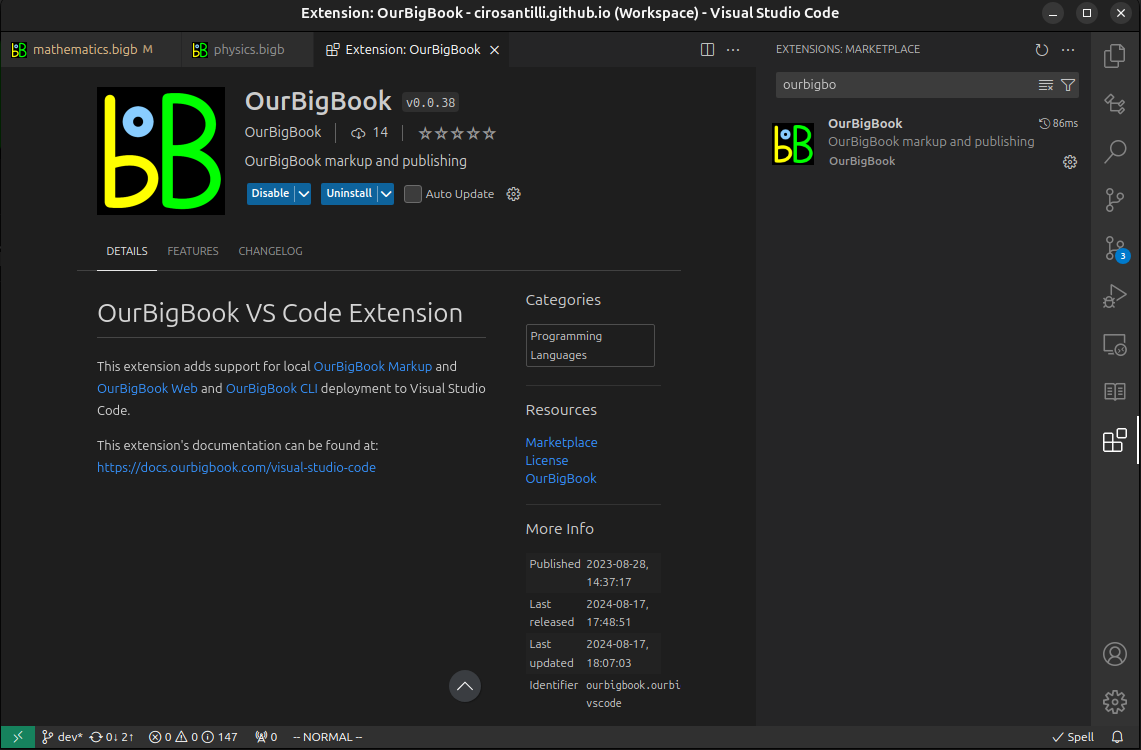
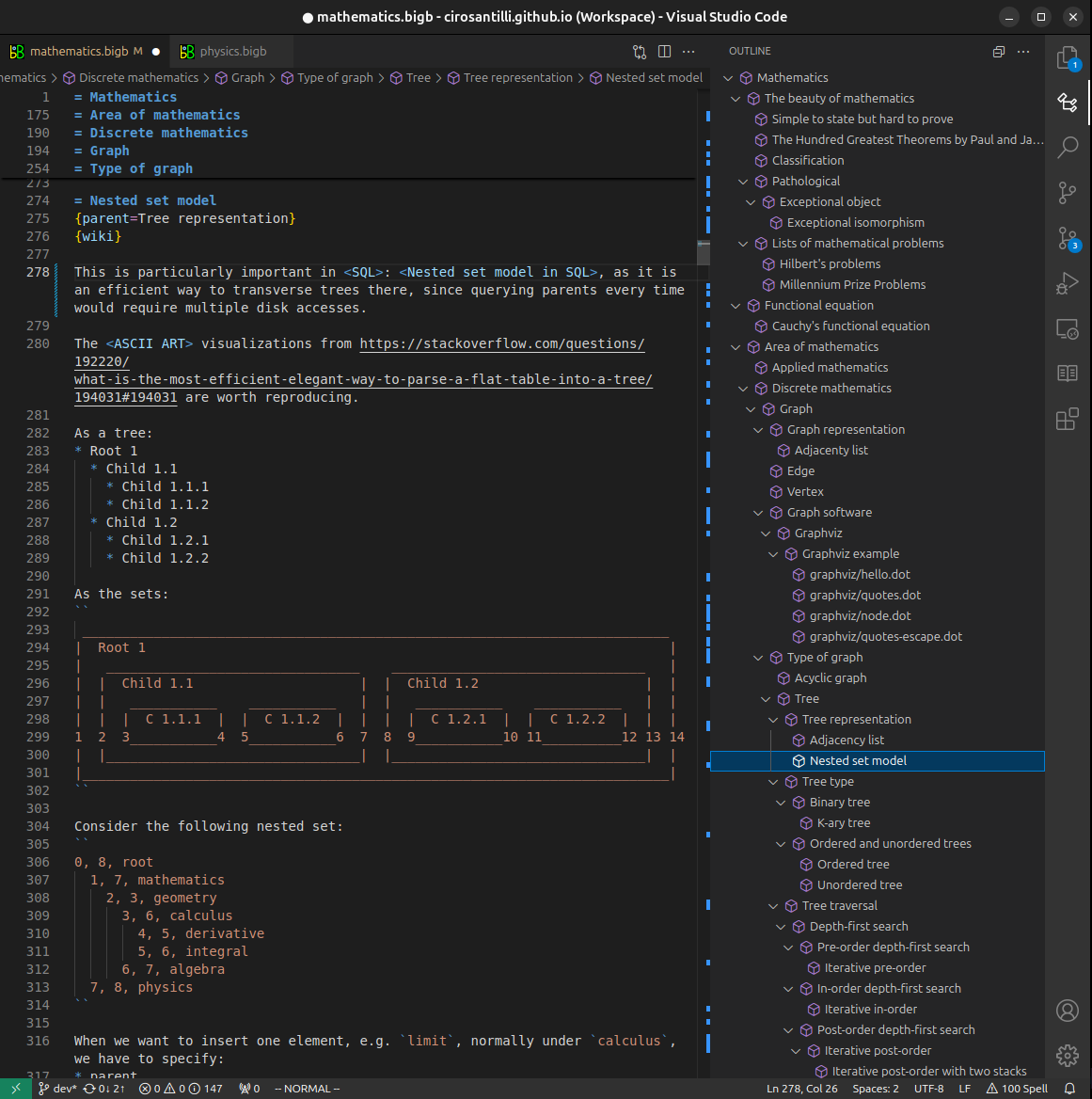
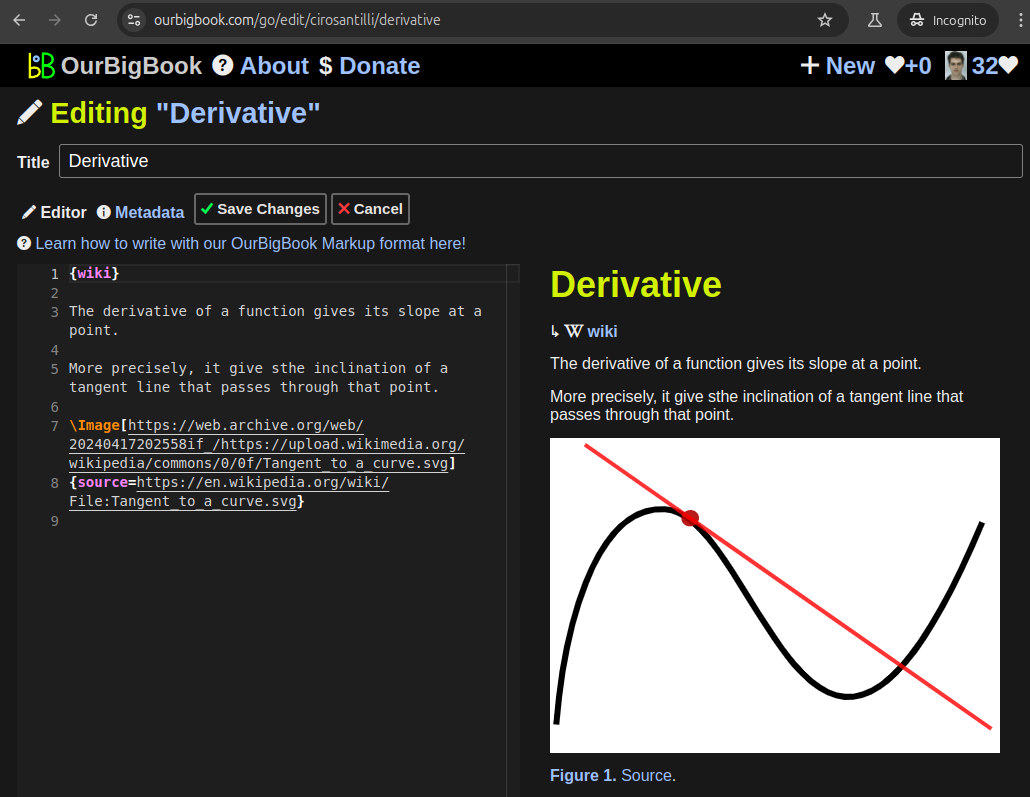
Figure 3. Visual Studio Code extension installation.Figure 4. Visual Studio Code extension tree navigation.Figure 5. Web editor. You can also edit articles on the Web editor without installing anything locally.Video 3. Edit locally and publish demo. Source. This shows editing OurBigBook Markup and publishing it using the Visual Studio Code extension.Video 4. OurBigBook Visual Studio Code extension editing and navigation demo. Source. - Infinitely deep tables of contents:
All our software is open source and hosted at: github.com/ourbigbook/ourbigbook
Further documentation can be found at: docs.ourbigbook.com
Feel free to reach our to us for any help or suggestions: docs.ourbigbook.com/#contact