Radiative transfer equation and diffusion theory for photon transport in biological tissue by  Wikipedia Bot 0
Wikipedia Bot 0
 Wikipedia Bot 0
Wikipedia Bot 0 The **radiative transfer equation (RTE)** is a fundamental equation that describes the propagation of radiation (such as light) through a medium. It considers the interactions of photons with matter, accounting for scattering and absorption processes, and is critical in understanding how light interacts with biological tissues.
The Cerebellar Model Articulation Controller (CMAC) is a type of neural network model inspired by the structure and function of the cerebellum in the human brain. It was developed for control and learning tasks, particularly in robotics and complex system simulations. ### Key Features of CMAC: 1. **Architecture**: - CMAC consists of a combination of memory storage and function approximation.
"Positively Bob: Willie Nile Sings Bob Dylan" is an album released by singer-songwriter Willie Nile, featuring his renditions of songs written by the iconic artist Bob Dylan. Released in 2021, the album showcases Nile's unique interpretations of Dylan's work, reflecting his own musical style while paying homage to Dylan's significant influence on rock and folk music. The title itself is a play on Dylan's song "Positively Fourth Street.
"Garage Classics" refers to a type of music or an event/venue that focuses on classic garage music, often rooted in the garage rock genre. Garage rock is a raw and energetic style of rock music that originated in the 1960s, characterized by its simple, lo-fi sound and often rebellious themes. It is closely associated with amateur bands and a DIY ethic.
Anders Lindquist might refer to several individuals, depending on the context, as it is a relatively common name. Without additional information, it is challenging to identify exactly who you are referring to. One notable Anders Lindquist is a Swedish professor known for his research in the field of computer science, particularly in areas like machine learning and data mining. If you provide more specifics about the context or field (science, sports, arts, etc.
ANDOS can refer to various things depending on the context, but one common interpretation is "Andos," which may refer to a type of mountain range or location in certain regions, particularly in reference to the Andes mountains in South America. However, it can also be an acronym or shorthand for specific organizations, processes, or technologies.
André Maréchal could refer to a few different individuals or topics, but without more specific context, it's difficult to pinpoint exactly what you're asking about. If you're referring to a notable figure, there might be individuals with that name in various fields such as arts, sports, or academia. Alternatively, it may relate to specific works or places named after a person named André Maréchal.
Post-transcriptional regulation refers to the control of gene expression at the RNA level after the transcription process, where the DNA is transcribed into messenger RNA (mRNA). This regulation can affect various stages of RNA processing and ultimately influence the amount and functionality of the resulting proteins.
A garden railway is a miniature railway system set up in a garden or outdoor area, typically using model trains that run on larger scales, such as G scale (1:22.5) or O scale (1:48). These railways can be designed to represent real-life tracks and scenery, including tunnels, bridges, and landscapes. Garden railways are often built for hobbyists who enjoy both gardening and model railroading.
"Panty Raid" is a studio album by the American hip-hop duo Tash Sultana and their frequent collaborator, Jay-1. Released in 2020, the album showcases a blend of various musical styles, including hip-hop, reggae, and R&B. The project features contributions from several artists and highlights the duo's distinct sound through a mixture of rhythmic beats and thought-provoking lyrics.
Anfinsen's dogma, named after biochemist Christian Anfinsen, refers to the principle that the three-dimensional structure of a protein is determined by its amino acid sequence. This concept emerged from Anfinsen's work in the 1960s, particularly his experiments with the enzyme ribonuclease A.
"Animal engines" typically refers to mechanisms or concepts that relate to the use of animals for power or labor, particularly in historical or agricultural contexts. Here are a couple of interpretations of the term: 1. **Historical Use of Animal Power**: In agriculture, "animal engines" may describe devices powered by animals, such as plows pulled by horses, mules, or oxen. These animals served as sources of energy for farming tasks and were vital before mechanization.
Annette Dobson is a notable figure in the field of statistics and biostatistics, particularly recognized for her work in epidemiology and public health. She has contributed to various research initiatives, focusing on issues such as disease prevention and health promotion.
Gary Gibbons is a prominent figure in the field of science communication, particularly known for his work in astronomy and planetary science. He has contributed to various educational programs and initiatives aimed at making complex scientific concepts accessible to the public. Gibbons is often associated with projects that involve public outreach in science, helping to foster greater understanding and interest in scientific topics among non-experts.
"Powers of Ten" is a short film released in 1977, directed by Charles and Ray Eames. It explores the relative scale of the universe by examining the effect of scale on the objects in the universe, from the very small to the very large. The film begins with a view of a picnic in Chicago and progressively zooms out by factors of ten, illustrating an expansive view of the cosmos.
A gas hydrate pingo, sometimes referred to as a gas hydrate mounds or gas hydrate structures, is a geological formation that occurs in polar and subpolar regions, primarily in permafrost or beneath ocean sediment. These formations are associated with the presence of gas hydrates, which are ice-like structures in which water molecules trap gas molecules, usually methane.
A. P. J. Abdul Kalam (Avul Pakir Jainulabdeen Abdul Kalam) was an Indian aerospace scientist and politician who served as the 11th President of India from 2002 to 2007. Born on October 15, 1931, in Rameswaram, Tamil Nadu, he played a pivotal role in India's space and missile development programs, earning the title "Missile Man of India.
Anti-nuclear protests refer to demonstrations and movements aimed at opposing the use of nuclear energy and nuclear weapons. These protests often arise from concerns over the environmental, health, safety, and ethical implications associated with nuclear technology. The anti-nuclear movement gained significant momentum in the latter half of the 20th century, particularly following nuclear accidents like the Three Mile Island incident in 1979, the Chernobyl disaster in 1986, and the Fukushima Daiichi nuclear disaster in 2011.
Antoine César Becquerel (1788–1878) was a French physicist and the grandfather of the more widely known Henri Becquerel. He is notable for his early work in the field of electricity and magnetism, along with contributions to the understanding of chemical processes and luminescence. During his career, he made various scientific contributions, including studies on phosphorescence and fluorescence.
Pinned article: Introduction to the OurBigBook Project
Welcome to the OurBigBook Project! Our goal is to create the perfect publishing platform for STEM subjects, and get university-level students to write the best free STEM tutorials ever.
Everyone is welcome to create an account and play with the site: ourbigbook.com/go/register. We belive that students themselves can write amazing tutorials, but teachers are welcome too. You can write about anything you want, it doesn't have to be STEM or even educational. Silly test content is very welcome and you won't be penalized in any way. Just keep it legal!
Intro to OurBigBook
. Source. We have two killer features:
- topics: topics group articles by different users with the same title, e.g. here is the topic for the "Fundamental Theorem of Calculus" ourbigbook.com/go/topic/fundamental-theorem-of-calculusArticles of different users are sorted by upvote within each article page. This feature is a bit like:
- a Wikipedia where each user can have their own version of each article
- a Q&A website like Stack Overflow, where multiple people can give their views on a given topic, and the best ones are sorted by upvote. Except you don't need to wait for someone to ask first, and any topic goes, no matter how narrow or broad
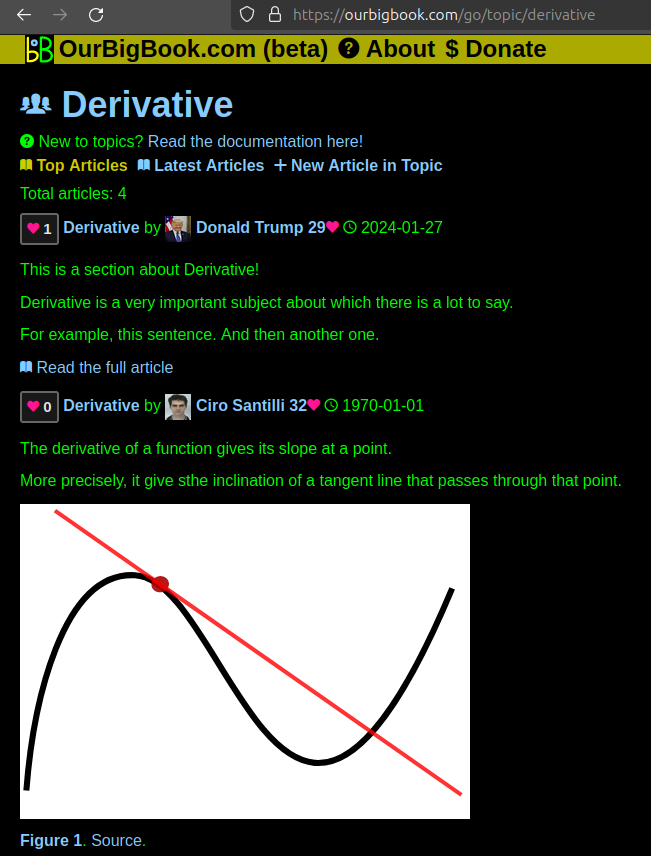
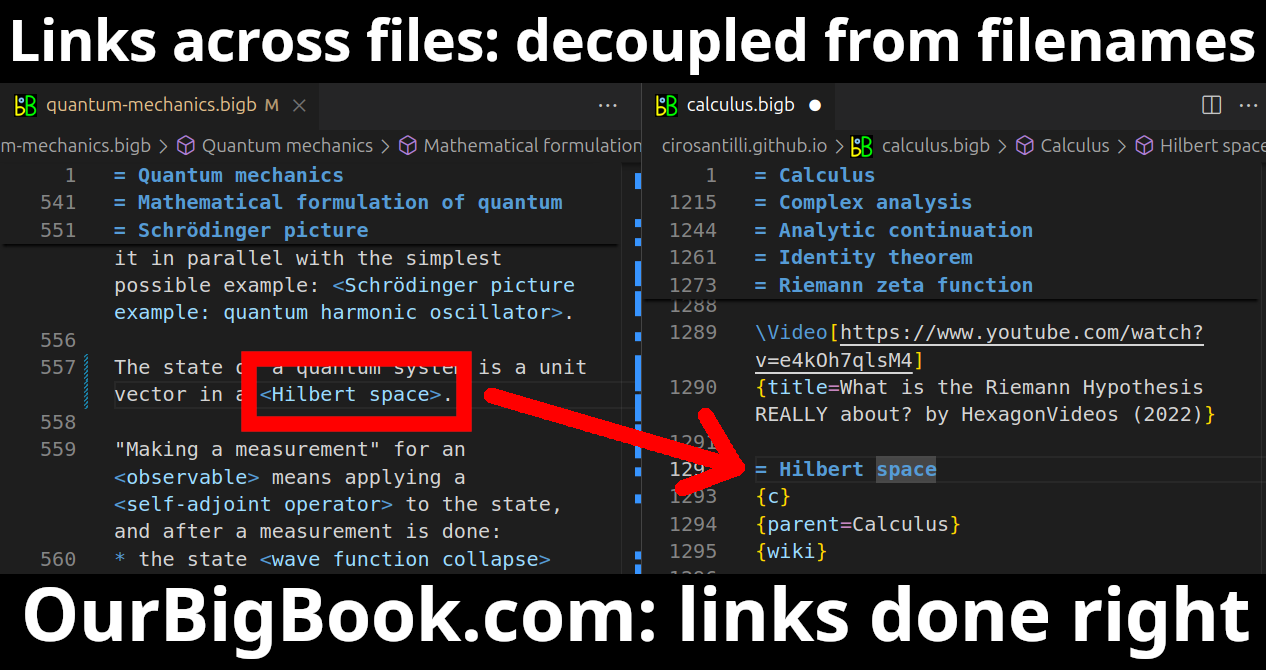
This feature makes it possible for readers to find better explanations of any topic created by other writers. And it allows writers to create an explanation in a place that readers might actually find it.Figure 1. Screenshot of the "Derivative" topic page. View it live at: ourbigbook.com/go/topic/derivativeVideo 2. OurBigBook Web topics demo. Source. - local editing: you can store all your personal knowledge base content locally in a plaintext markup format that can be edited locally and published either:This way you can be sure that even if OurBigBook.com were to go down one day (which we have no plans to do as it is quite cheap to host!), your content will still be perfectly readable as a static site.
- to OurBigBook.com to get awesome multi-user features like topics and likes
- as HTML files to a static website, which you can host yourself for free on many external providers like GitHub Pages, and remain in full control
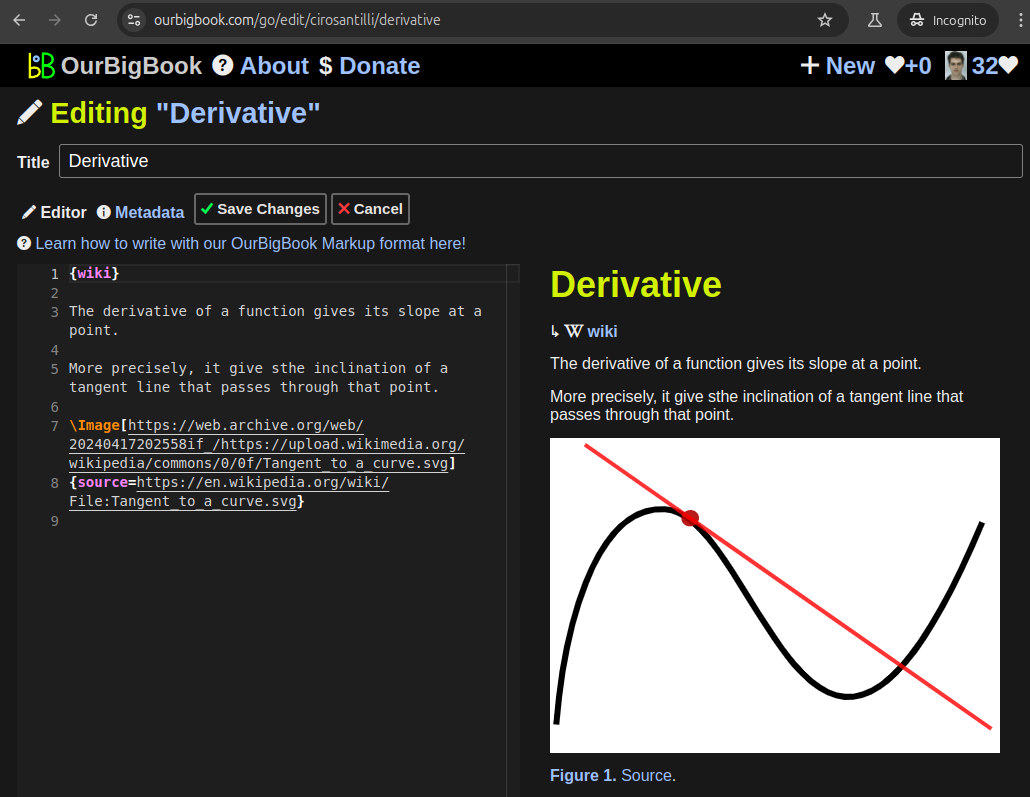
Figure 3. Visual Studio Code extension installation.Figure 4. Visual Studio Code extension tree navigation.Figure 5. Web editor. You can also edit articles on the Web editor without installing anything locally.Video 3. Edit locally and publish demo. Source. This shows editing OurBigBook Markup and publishing it using the Visual Studio Code extension.Video 4. OurBigBook Visual Studio Code extension editing and navigation demo. Source. - Infinitely deep tables of contents:
All our software is open source and hosted at: github.com/ourbigbook/ourbigbook
Further documentation can be found at: docs.ourbigbook.com
Feel free to reach our to us for any help or suggestions: docs.ourbigbook.com/#contact