The Maximum-Entropy Markov Model (MEMM) is a type of statistical model used for sequence prediction tasks, particularly in the fields of natural language processing (NLP) and bioinformatics. It combines concepts from maximum entropy modeling and Markov models to make predictions about sequential data.
A McLeod gauge is an instrument used to measure very low pressures, particularly in the range of 0.1 to 10^-6 torr (or approximately 0.1 to 10^-6 mmHg). It operates based on the principle of gas compression and relies on Gay-Lussac's law of gas behavior. The device consists of a small volume of gas that is compressed into a manometer tube.
Mediation in statistics refers to a statistical analysis technique that seeks to understand the process or mechanism through which one variable (the independent variable) influences another variable (the dependent variable) via a third variable (the mediator). Essentially, mediation helps to explore and explain the relationship between variables by examining the role of the mediator. Here’s a breakdown of the concepts involved: 1. **Independent Variable (IV)**: This is the variable that is presumed to cause an effect.
The metric system is an international system of measurement that is based on the decimal system. It is used for scientific, industrial, and everyday measurements in most countries around the world. The metric system is designed to be simple and logical, with units that are related by factors of ten, making it easy to convert between them.
Medieval Syrian astronomers played a significant role in the development of astronomy during the medieval period, particularly from the 7th to the 14th centuries. During this time, the Middle East, including regions that are now part of modern Syria, was a center of scholarship and learning, influenced by various cultures and traditions, including Greek, Persian, and later Islamic scholarship.
Medieval weather events refer to significant climatic and weather-related occurrences during the Middle Ages, roughly spanning from the 5th to the late 15th century. This period experienced a variety of weather-related phenomena, many of which had profound impacts on agriculture, population, and society.
Mel-frequency cepstrum (MFC) is a representation of the short-term power spectrum of a sound signal that is commonly used in the fields of speech and audio processing. It is particularly important in applications such as speech recognition, speaker identification, and other areas involving acoustic signal analysis. ### Key Concepts: 1. **Mel Scale**: - The Mel scale is a perceptual scale of pitches that approximates the way humans perceive sound frequencies.
A meteotsunami is a series of ocean waves that are generated by meteorological phenomena, rather than by seismic activity, such as earthquakes. These waves are typically caused by rapid changes in atmospheric pressure or strong winds over a body of water, which can create disturbances in the water surface. Meteotsunamis are often characterized by their short wave periods, typically ranging from a few minutes to an hour.
The Merge algorithm is a fundamental algorithm often used in the context of sorting, specifically within the Merge Sort algorithm. Merge Sort is a divide-and-conquer algorithm that sorts an array or list by dividing it into smaller subarrays, sorting those subarrays, and then merging them back together into a single sorted array. ### Merge Algorithm Overview: 1. **Divide**: The input array is split into two halves until each subarray contains a single element.
Metadata modeling is the process of creating a structured framework that defines the metadata—data about data—associated with various data elements within a system, database, or information repository. The purpose of metadata modeling is to improve the understanding, management, and usability of data by providing clear insights into what the data represents, its relationships, and how it can be used.
"Mexican statisticians" could refer to statisticians from Mexico or individuals who specialize in statistical methods and data analysis within the field of statistics in Mexico. Like statisticians from other countries, they play a crucial role in various sectors, including academia, government, industry, health, and social sciences, contributing to the analysis and interpretation of data to inform decision-making and research.
In Mexico, the official system of measurement is the metric system, which includes units such as meters for distance, kilograms for weight, and liters for volume. Here are some common metric units used in Mexico: 1. **Length**: - Meter (m) - Centimeter (cm) - Kilometer (km) 2. **Mass**: - Kilogram (kg) - Gram (g) 3.
Michel Campillo is likely a French geologist known for his contributions to the study of seismic activity and earthquake phenomena. He has been associated with various research works focusing on the interpretation of seismic data and understanding the behavior of earthquakes. His work has implications for seismic risk assessment and understanding geological processes.
MIMOS II refers to the Malaysian Institute of Microelectronics Systems II, which is a significant initiative in Malaysia aimed at advancing research, development, and innovation in the field of microelectronics and related technology. The MIMOS II project is part of Malaysia's broader efforts to develop its indigenous capabilities in semiconductor technologies and to promote research in areas such as microelectronics, materials science, and nanotechnology.
London offers a diverse array of transport options to navigate the city efficiently. Here are the primary modes of transport available: 1. **London Underground (Tube)**: A vast network of trains serving the city and its suburbs, the Tube is one of the fastest ways to travel around London. 2. **Buses**: London has an extensive bus network, with iconic red double-decker buses operating throughout the city, providing a scenic way to see the sights.
Molecular graphics is a field that involves the visualization of molecular structures and complexes using computer-generated imagery. It plays a crucial role in biochemistry, molecular biology, and drug design by allowing researchers to create, manipulate, and analyze three-dimensional representations of molecules. Key aspects of molecular graphics include: 1. **Visualization**: It enables scientists to visualize complex molecular structures, such as proteins, nucleic acids, and small molecules, in a way that is easily interpretable.
Molecular-scale temperature refers to the average kinetic energy of molecules within a substance at a microscopic level. In thermodynamics, temperature is often defined as a measure of the thermal energy of a system. At the molecular scale, temperature correlates with how fast the molecules are moving: the higher the temperature, the faster the molecules move. Within a gas, for example, molecular-scale temperature reflects the average speed of the gas molecules.
Monsieur et Madame jokes are a form of humor that originated in France, featuring playful puns based on names. Each joke usually follows a structure where a character named "Monsieur" or "Madame" has a last name that reflects a particular trait or profession, leading to a humorous or ironic situation. For example: - **Monsieur et Madame Pomme** have a daughter named **Charlotte** (referring to "Charlotte aux pommes," which means "apple tart").
A "mother ship" typically refers to a large vessel or aircraft that serves as a base or support platform for smaller vessels or aircraft. The term is commonly used in various contexts: 1. **Naval and Air Force Context**: In military or naval operations, a mother ship can be a large ship that deploys smaller ships, submarines, or aircraft to carry out missions. For example, an aircraft carrier acts as a mother ship for fighter jets.
The Motion Picture Patents Company (MPPC), often referred to as the Edison Trust, was a trust formed in 1908 by several major film producers and the Edison Manufacturing Company, which was controlled by Thomas Edison. The primary purpose of the MPPC was to monopolize the production and distribution of films in the United States, effectively controlling the movie industry during the early years of cinema.
Pinned article: Introduction to the OurBigBook Project
Welcome to the OurBigBook Project! Our goal is to create the perfect publishing platform for STEM subjects, and get university-level students to write the best free STEM tutorials ever.
Everyone is welcome to create an account and play with the site: ourbigbook.com/go/register. We belive that students themselves can write amazing tutorials, but teachers are welcome too. You can write about anything you want, it doesn't have to be STEM or even educational. Silly test content is very welcome and you won't be penalized in any way. Just keep it legal!
Intro to OurBigBook
. Source. We have two killer features:
- topics: topics group articles by different users with the same title, e.g. here is the topic for the "Fundamental Theorem of Calculus" ourbigbook.com/go/topic/fundamental-theorem-of-calculusArticles of different users are sorted by upvote within each article page. This feature is a bit like:
- a Wikipedia where each user can have their own version of each article
- a Q&A website like Stack Overflow, where multiple people can give their views on a given topic, and the best ones are sorted by upvote. Except you don't need to wait for someone to ask first, and any topic goes, no matter how narrow or broad
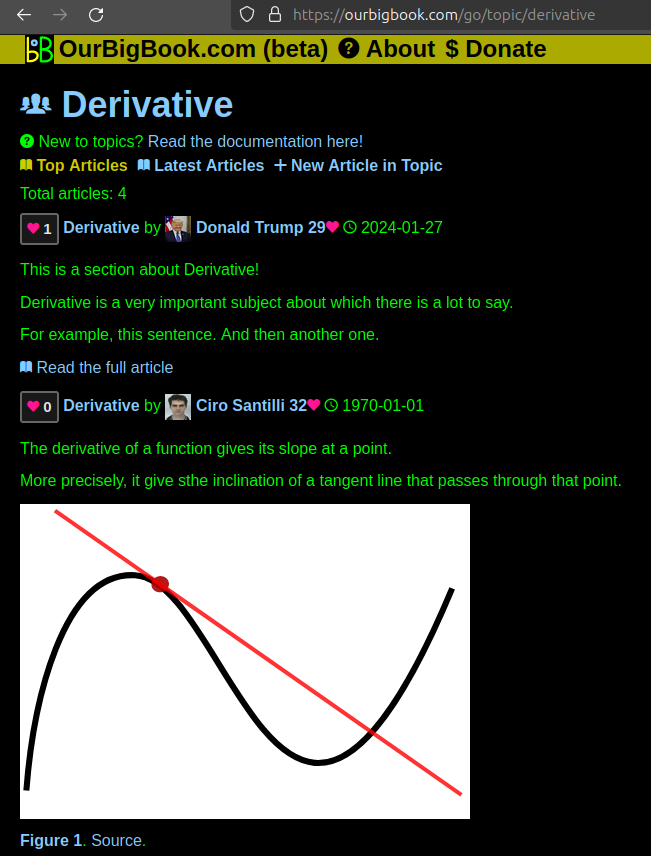
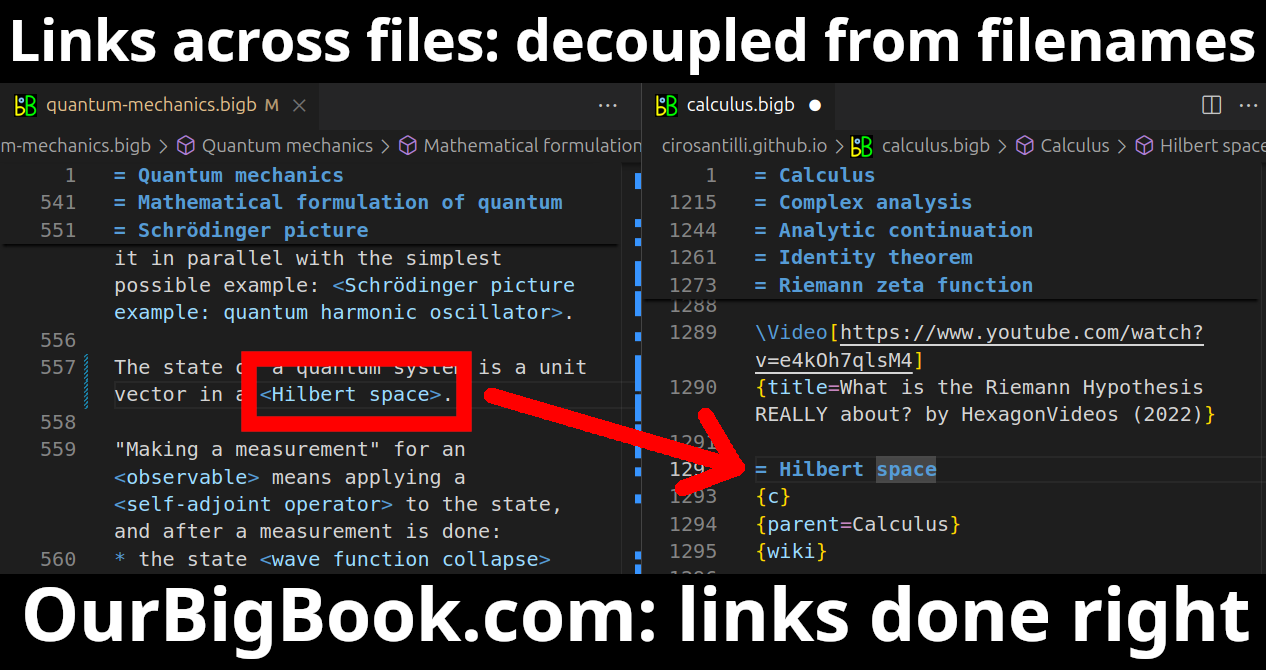
This feature makes it possible for readers to find better explanations of any topic created by other writers. And it allows writers to create an explanation in a place that readers might actually find it.Figure 1. Screenshot of the "Derivative" topic page. View it live at: ourbigbook.com/go/topic/derivativeVideo 2. OurBigBook Web topics demo. Source. - local editing: you can store all your personal knowledge base content locally in a plaintext markup format that can be edited locally and published either:This way you can be sure that even if OurBigBook.com were to go down one day (which we have no plans to do as it is quite cheap to host!), your content will still be perfectly readable as a static site.
- to OurBigBook.com to get awesome multi-user features like topics and likes
- as HTML files to a static website, which you can host yourself for free on many external providers like GitHub Pages, and remain in full control
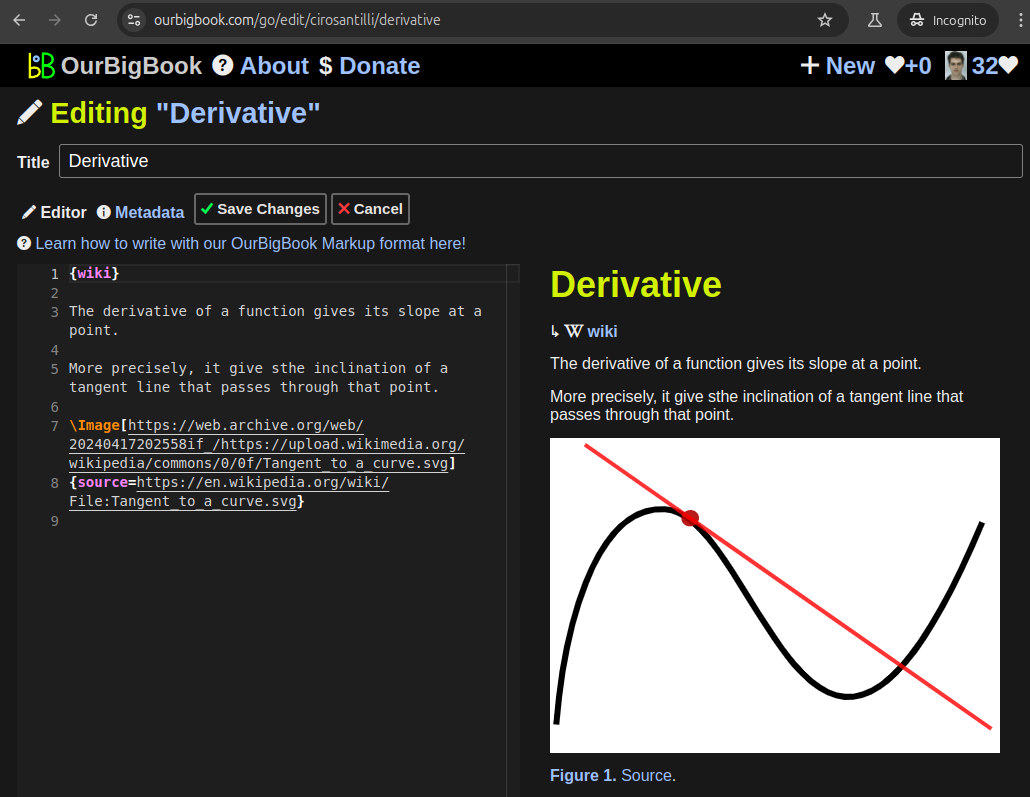
Figure 3. Visual Studio Code extension installation.Figure 4. Visual Studio Code extension tree navigation.Figure 5. Web editor. You can also edit articles on the Web editor without installing anything locally.Video 3. Edit locally and publish demo. Source. This shows editing OurBigBook Markup and publishing it using the Visual Studio Code extension.Video 4. OurBigBook Visual Studio Code extension editing and navigation demo. Source. - Infinitely deep tables of contents:
All our software is open source and hosted at: github.com/ourbigbook/ourbigbook
Further documentation can be found at: docs.ourbigbook.com
Feel free to reach our to us for any help or suggestions: docs.ourbigbook.com/#contact