Chemistry is fun. Too hard for precise physics (pre quantum computing, see also quantum chemistry), but not too hard for some maths like social sciences.
And it underpins biology.
Much before atoms were thought to be "experimentally real", chemists from the 19th century already used "conceptual atoms" as units for the proportions observed in macroscopic chemical reactions, e.g. . The thing is, there was still the possibility that those proportions were made up of something continuous that for some reason could only combine in the given proportions, so the atoms could only be strictly consider calculatory devices pending further evidence.
Subtle is the Lord by Abraham Pais (1982) chapter 5 "The reality of molecules" has some good mentions. Notably, physicists generally came to believe in atoms earlier than chemists, because the phenomena they were most interested in, e.g. pressure in the ideal gas law, and then Maxwell-Boltzmann statistics just scream atoms more loudly than chemical reactions, as they saw that these phenomena could be explained to some degree by traditional mechanics of little balls.
Confusion around the probabilistic nature of the second law of thermodynamics was also used as a physical counterargument by some. Pais mentions that Wilhelm Ostwald notably argued that the time reversibility of classical mechanics + the second law being a fundamental law of physics (and not just probabilistic, which is the correct hypothesis as we now understand) must imply that atoms are not classic billiard balls, otherwise the second law could be broken.
Pais also mentions that a big "chemical" breakthrough was isomers suggest that atoms exist.
Very direct evidence evidence:
- Brownian motion mathematical analysis in 1908. Brownian motion just makes it too clear that liquids cannot be continuous... if they were, there would obviously be no Brownian motion, full stop.
- X-ray crystallography: it sees crystal latices
Less direct evidence:
- 1874 Isomers suggest that atoms exist
- kinetic theory of gases seems to explain certain phenomena really well
Subtle is the Lord by Abraham Pais (1982) page 40 mentions several methods that Einstein used to "prove" that atoms were real. Perhaps the greatest argument of all is that several unrelated methods give the same estimates of atom size/mass:
- from 1905:
- in light quantum paper
- enabled by experimental work of Wilhelm Pfeffer on producing rigid membranes
- 1911: blueness of the sky and critical opalescence
Subtle is the Lord by Abraham Pais (1982) mentions that this has a good summary of the atomic theory evidence that was present at the time, and which had become basically indisputable at or soon after that date.
On Wikimedia Commons since it is now public domain in most countries: commons.wikimedia.org/w/index.php?title=File:Perrin,_Jean_-_Les_Atomes,_F%C3%A9lix_Alcan,_1913.djvu
An English translation from 1916 by English chemist Dalziel Llewellyn Hammick on the Internet Archive, also on the public domain: archive.org/details/atoms00hammgoog
Subtle is the Lord by Abraham Pais (1982) page 85:so it is quite cool to see that organic chemistry is one of the things that pushed atomic theory forward. Because when you start to observe that isomers has different characteristics, despite identical proportions of atoms, this is really hard to explain without talking about the relative positions of the atoms within molecules!
However, it became increasingly difficult in chemical circles to deny the reality of molecules after 1874, the year in which Jacobus Henricus van't Hoff and Joseph Achille Le Bel independently explained the isomerism of certain organic substances in terms of stereochemical properties of carbon compounds.
Was the first model to explain the Balmer series, notably linking atomic spectra to the Planck constant and therefore to other initial quantum mechanical observations.
This was one of the first major models that just said:
I give up, I can't tie this to classical physics in any way, let's just roll with it, OK?
It still treats electrons as little points spinning around the nucleus, but it makes the non-classical postulate that only certain angular momentums (and therefore energies) are allowed.
Bibliography:
- Inward Bound by Abraham Pais (1988) Chapter 9.e Atomic structure and spectral lines - Niels Bohr
- The Quantum Story by Jim Baggott (2011) Chapter 3 A Little Bit of Reality
Bagic jump between orbitals in the Bohr model. Analogous to the later wave function collapse in the Schrödinger equation.
Refinement of the Bohr model that starts to take quantum angular momentum into account in order to explain missing lines that would have been otherwise observed TODO specific example of such line.
They are not observe because they would violate the conservation of angular momentum.
Introduces the azimuthal quantum number and magnetic quantum number.
This technique is crazy! It allows to both:You actually see discrete peaks at different minute counts on the other end.
- separate gaseous mixtures
- identify gaseous compounds
It is based on how much the gas interacts with the column.
Detection is usually done burning the sample to ionize it when it comes out, and then you measure the current produced.
Gas chromatography by Quick Biochemistry Basics (2019)
Source. Based on the fact that we don't have a P algorithm for integer factorization as of 2020. But nor proof that one does not exist!
The private key is made of two randomly generated prime numbers: and . How such large primes are found: how large primes are found for RSA.
The public key is made of:
n = p*q- a randomly chosen integer exponent between
1ande_max = lcm(p -1, q -1), wherelcmis the Least common multiple
Given a plaintext message This operation is called modular exponentiation can be calculated efficiently with the Extended Euclidean algorithm.
m, the encrypted ciphertext version is:c = m^e mod nThe inverse operation of finding the private
m from the public c, e and is however believed to be a hard problem without knowing the factors of n.Bibliography:
- www.comparitech.com/blog/information-security/rsa-encryption/ has a numeric example
Pinned article: Introduction to the OurBigBook Project
Welcome to the OurBigBook Project! Our goal is to create the perfect publishing platform for STEM subjects, and get university-level students to write the best free STEM tutorials ever.
Everyone is welcome to create an account and play with the site: ourbigbook.com/go/register. We belive that students themselves can write amazing tutorials, but teachers are welcome too. You can write about anything you want, it doesn't have to be STEM or even educational. Silly test content is very welcome and you won't be penalized in any way. Just keep it legal!
Intro to OurBigBook
. Source. We have two killer features:
- topics: topics group articles by different users with the same title, e.g. here is the topic for the "Fundamental Theorem of Calculus" ourbigbook.com/go/topic/fundamental-theorem-of-calculusArticles of different users are sorted by upvote within each article page. This feature is a bit like:
- a Wikipedia where each user can have their own version of each article
- a Q&A website like Stack Overflow, where multiple people can give their views on a given topic, and the best ones are sorted by upvote. Except you don't need to wait for someone to ask first, and any topic goes, no matter how narrow or broad
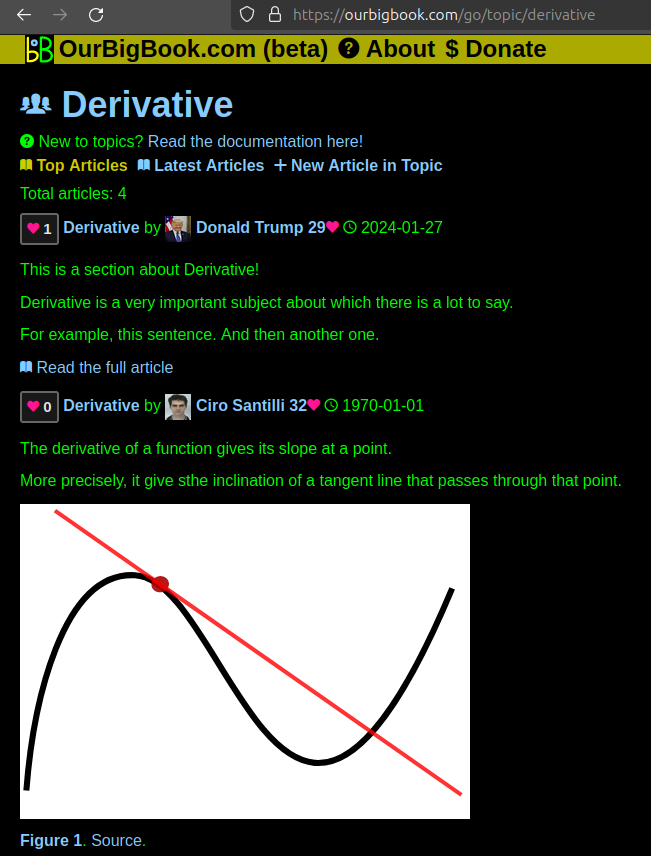
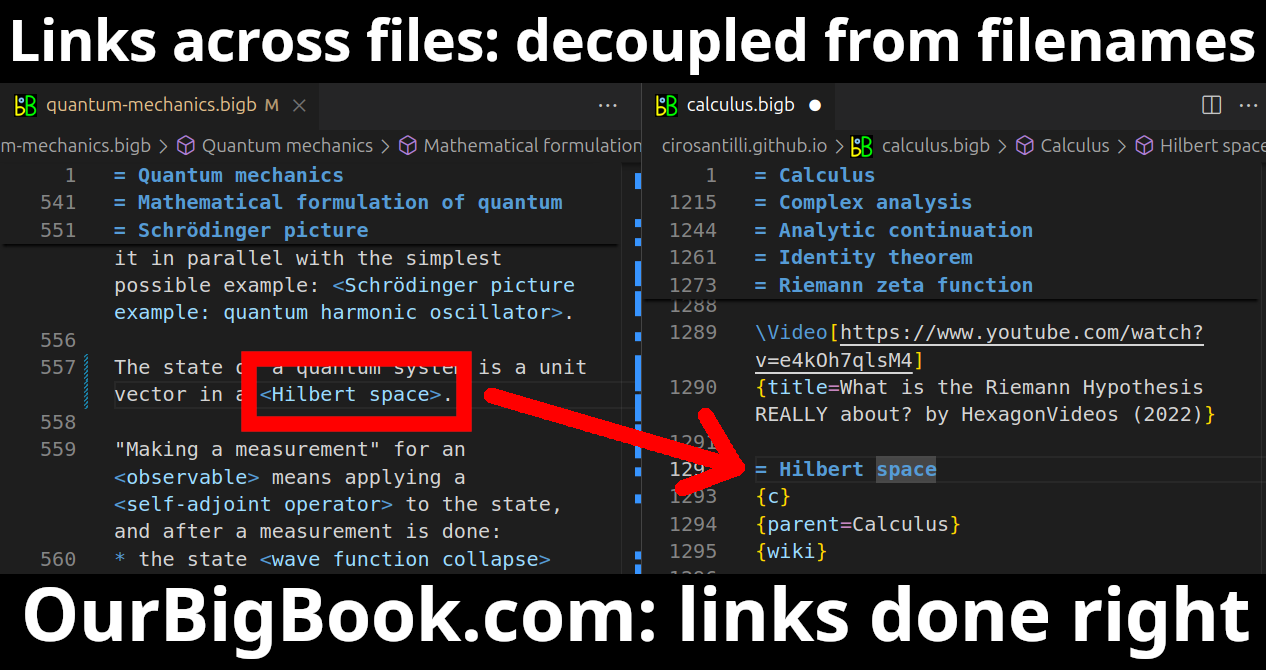
This feature makes it possible for readers to find better explanations of any topic created by other writers. And it allows writers to create an explanation in a place that readers might actually find it.Figure 1. Screenshot of the "Derivative" topic page. View it live at: ourbigbook.com/go/topic/derivativeVideo 2. OurBigBook Web topics demo. Source. - local editing: you can store all your personal knowledge base content locally in a plaintext markup format that can be edited locally and published either:This way you can be sure that even if OurBigBook.com were to go down one day (which we have no plans to do as it is quite cheap to host!), your content will still be perfectly readable as a static site.
- to OurBigBook.com to get awesome multi-user features like topics and likes
- as HTML files to a static website, which you can host yourself for free on many external providers like GitHub Pages, and remain in full control
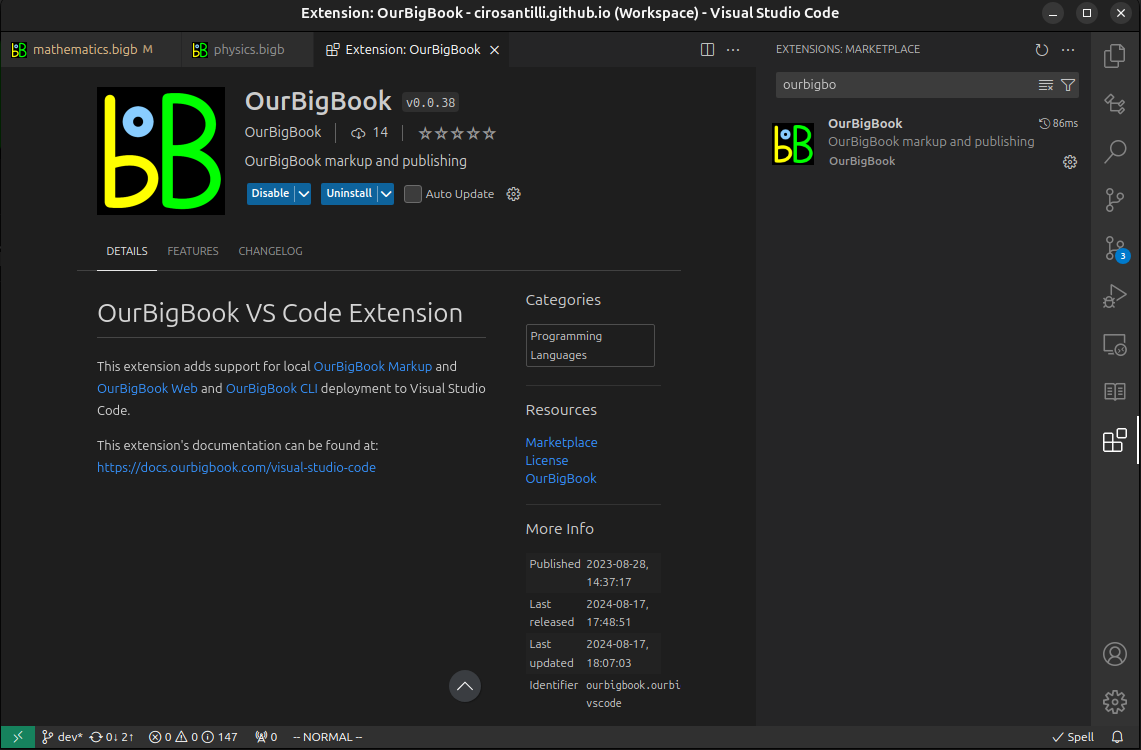
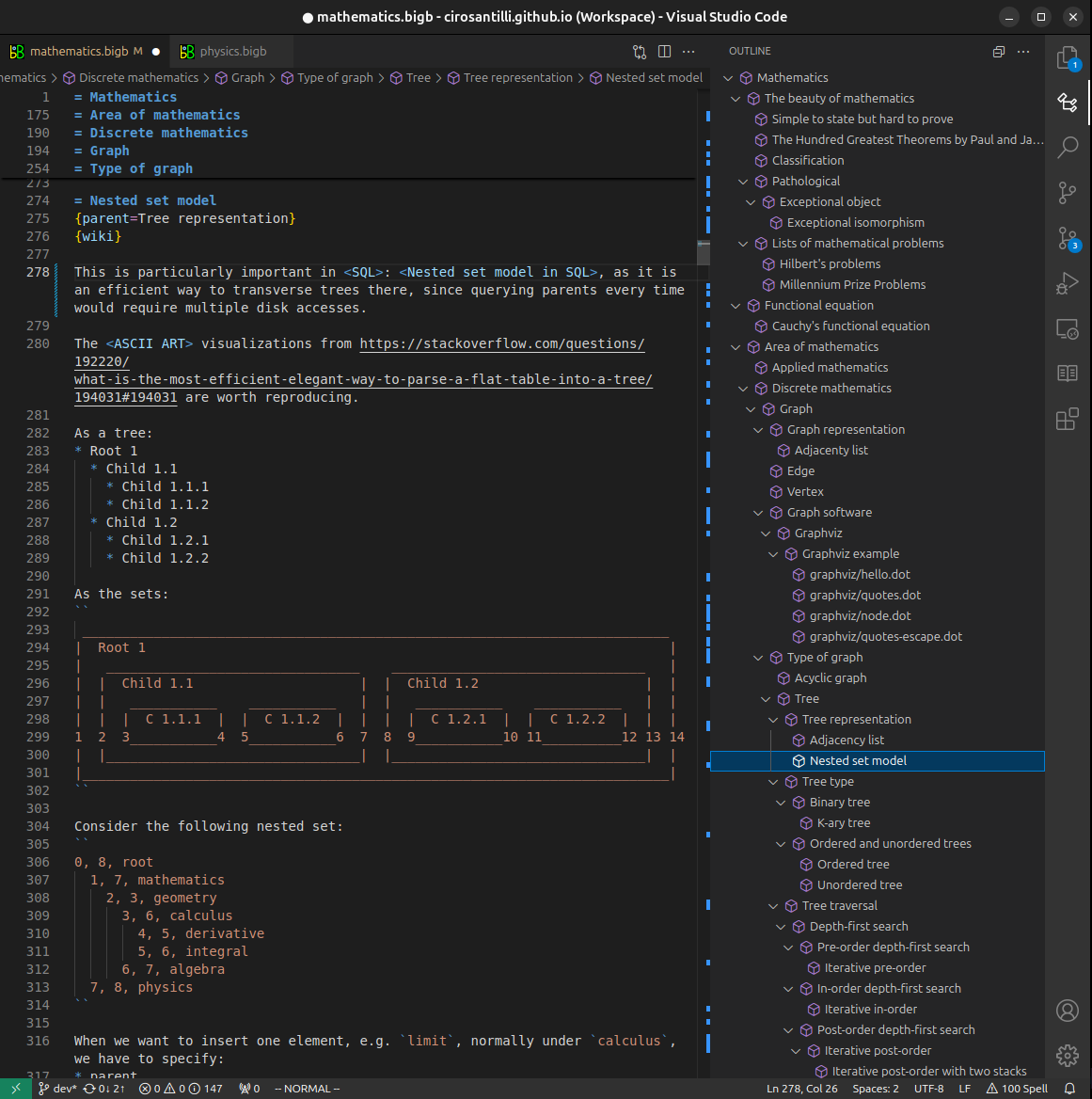
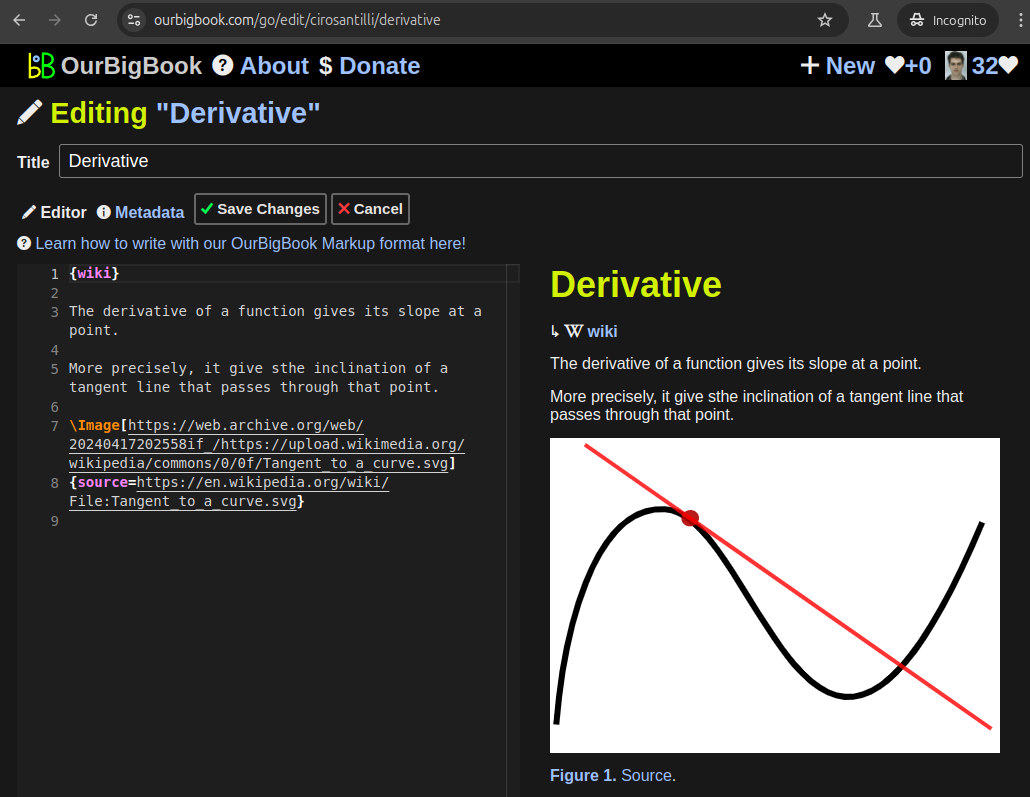
Figure 3. Visual Studio Code extension installation.Figure 4. Visual Studio Code extension tree navigation.Figure 5. Web editor. You can also edit articles on the Web editor without installing anything locally.Video 3. Edit locally and publish demo. Source. This shows editing OurBigBook Markup and publishing it using the Visual Studio Code extension.Video 4. OurBigBook Visual Studio Code extension editing and navigation demo. Source. - Infinitely deep tables of contents:
All our software is open source and hosted at: github.com/ourbigbook/ourbigbook
Further documentation can be found at: docs.ourbigbook.com
Feel free to reach our to us for any help or suggestions: docs.ourbigbook.com/#contact