Last updated: 2013.
There were apparently some lecture videos at: web.archive.org/web/20030604194654/http://physicsstream.ucsd.edu/courses/spring2003/physics130a/ as pointed out by Matthew Heaney[ref], .mov files can be found at: web.archive.org/web/*/http://physicsstream.ucsd.edu/courses/spring2003/physics130a/*, but we were yet unable to open them, related:
These are the key mathematical ideas to understand!!
There are actually a few formulations out there. By far the dominant one as of 2020 has been the Schrödinger picture, which contrasts notably with the Heisenberg picture.
Another well known one is the de Broglie-Bohm theory, which is deterministic, but non-local.
Caller ID spoofing is the technique of artificially manipulating the phone number that appears on the recipient's caller ID display. This can be done for a variety of reasons, both legitimate and illegitimate. ### How It Works When a call is made, the phone network typically transmits the calling party's number, which the receiving party sees on their caller ID.
Carl August von Schmidt (1822–1892) was a German architect best known for his significant contributions to the architectural landscape of the 19th century, particularly in Germany. His work encompassed a variety of building types, including churches, civic buildings, and residential structures. He often utilized elements of the Neoclassical and Historicist styles, which were popular during his time.
Trouton's rule is a principle in physical chemistry that provides an estimate for the entropy of vaporization of a liquid. It states that the entropy of vaporization (\( \Delta S_{vap} \)) of many liquids at their normal boiling points is approximately equal to a constant value, which is about 88 to 100 J/mol·K. This rule holds true for a variety of organic liquids, particularly those that are non-polar or weakly polar.
Cather Simpson is a renowned physicist and a professor known for her work in ultrafast optics and photonics. She has conducted significant research in the field of laser science and its applications, particularly in studying light-matter interactions on extremely short timescales. In addition to her scientific contributions, Cather Simpson is recognized for her commitment to science communication and education, aiming to inspire the next generation of scientists.
Athanase Dupré is a notable figure in the field of French art, particularly recognized for his contributions as a painter and artist in the 19th century. He is often associated with the Barbizon School, which emphasized naturalism and landscape painting. His work is characterized by a focus on the beauty of nature and the depiction of rural life.
"Causality" is a book by Judea Pearl, published in 2000, that presents a comprehensive analysis of causal reasoning and its implications in various fields such as statistics, artificial intelligence, and philosophy. Pearl, a prominent figure in the field of artificial intelligence, introduces a framework for understanding causation that goes beyond traditional correlation-based approaches. In the book, Pearl discusses the importance of distinguishing between correlation and causation, providing tools and methodologies for reasoning about causality.
The term "Centered World" might refer to various concepts depending on the context in which it is used. Without more context, it's difficult to provide a definitive answer, as "Centered World" doesn't correspond to a widely recognized concept in fields like psychology, sociology, philosophy, or geography. However, here are a few potential interpretations of the term: 1. **Philosophical or Psychological Context**: It could refer to a state of mental or emotional equilibrium.
Public transport by mode refers to the various types of transportation systems available for public use, categorized based on the mode of travel. Here’s an overview of the primary modes of public transport: 1. **Buses:** - Buses are a common mode of public transport that operate on fixed routes and schedules. They can serve cities, towns, and rural areas and are often cost-effective and accessible.
The Čech-to-derived functor spectral sequence is a tool in homological algebra and sheaf theory that relates Čech cohomology to derived functors, particularly sheaf cohomology. This spectral sequence emerges in contexts where one is interested in understanding the relationship between local properties, codified by Čech cohomology, and global properties captured by derived functors like the derived functors of sheaf cohomology. ### Overview of the Components Involved 1.
Puerto Rico, as a territory of the United States, primarily uses the imperial system of measurement, which includes units such as inches, feet, and pounds. This is consistent with the measurements used in the mainland U.S. However, the metric system is also widely recognized and used, particularly in scientific, educational, and medical contexts. In everyday life, Puerto Ricans will commonly express distances in miles, height in feet and inches, and weights in pounds.
Pulse-density modulation (PDM) is a form of modulation used to represent an analog signal with a binary signal. In PDM, the density of the pulses corresponds to the amplitude of the analog signal being represented. Essentially, the more frequent the pulses occur in a given time frame, the higher the average value of the analog signal.
Champion is a well-known brand that manufactures spark plugs and other ignition system components for various types of engines, including those found in automobiles, motorcycles, lawnmowers, and industrial equipment. Founded in 1907, Champion has a long history of innovation in spark plug technology and is recognized for producing high-quality products that meet the needs of both consumers and professionals. Spark plugs are essential components in internal combustion engines, as they ignite the air-fuel mixture in the engine's cylinders.
George Sugihara is an American mathematician and a prominent figure in the field of mathematical biology and ecology. He is best known for his work in mathematical modeling and the application of mathematical techniques to understand complex ecological systems, including population dynamics and species interactions. Sugihara has contributed significantly to the development of methods for analyzing time series data in ecological research, and he has worked on various projects related to biodiversity and the stability of ecosystems.
As of my last update in October 2023, there is no widely recognized figure, concept, or event specifically known as "Charles Cadwell." It's possible that the name could refer to a private individual, a fictional character, or a less-publicized person or subject that has emerged after my last training data.
Chirp compression is a signal processing technique often used in various fields, including radar and sonar systems, communication technologies, and audio processing. It involves the use of frequency-modulated signals, typically called "chirps," which are signals whose frequency increases or decreases over time. The basic concept of chirp compression is to improve the signal-to-noise ratio and enhance the detection capabilities of the signal by shaping it in a way that allows for better resolution and clarity when the signal is processed.
Christine Darden is an American mathematician, aerospace engineer, and author known for her significant contributions to the fields of aerodynamics and the aeronautics industry. She was part of the team at NASA Langley Research Center, where she worked on developing supersonic and hypersonic flight technologies and played a vital role in addressing issues related to sonic booms.
Pinned article: Introduction to the OurBigBook Project
Welcome to the OurBigBook Project! Our goal is to create the perfect publishing platform for STEM subjects, and get university-level students to write the best free STEM tutorials ever.
Everyone is welcome to create an account and play with the site: ourbigbook.com/go/register. We belive that students themselves can write amazing tutorials, but teachers are welcome too. You can write about anything you want, it doesn't have to be STEM or even educational. Silly test content is very welcome and you won't be penalized in any way. Just keep it legal!
Intro to OurBigBook
. Source. We have two killer features:
- topics: topics group articles by different users with the same title, e.g. here is the topic for the "Fundamental Theorem of Calculus" ourbigbook.com/go/topic/fundamental-theorem-of-calculusArticles of different users are sorted by upvote within each article page. This feature is a bit like:
- a Wikipedia where each user can have their own version of each article
- a Q&A website like Stack Overflow, where multiple people can give their views on a given topic, and the best ones are sorted by upvote. Except you don't need to wait for someone to ask first, and any topic goes, no matter how narrow or broad
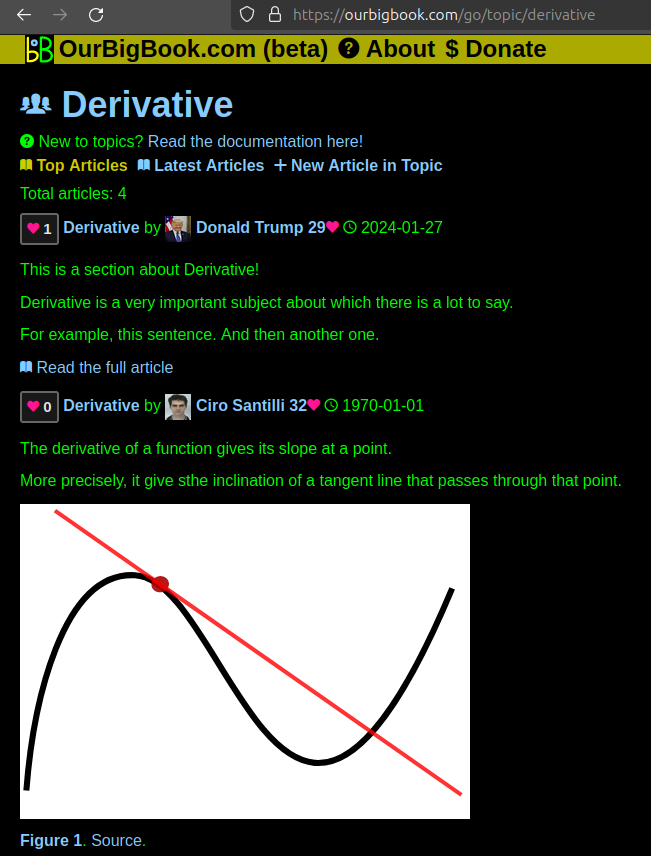
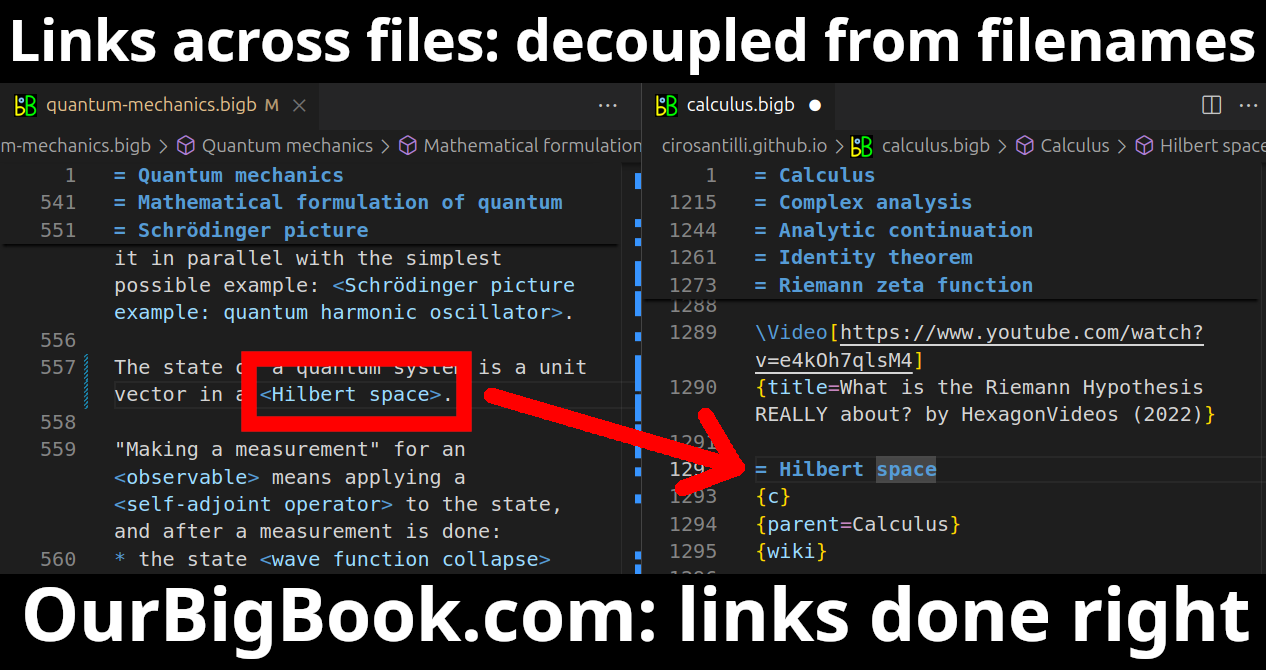
This feature makes it possible for readers to find better explanations of any topic created by other writers. And it allows writers to create an explanation in a place that readers might actually find it.Figure 1. Screenshot of the "Derivative" topic page. View it live at: ourbigbook.com/go/topic/derivativeVideo 2. OurBigBook Web topics demo. Source. - local editing: you can store all your personal knowledge base content locally in a plaintext markup format that can be edited locally and published either:This way you can be sure that even if OurBigBook.com were to go down one day (which we have no plans to do as it is quite cheap to host!), your content will still be perfectly readable as a static site.
- to OurBigBook.com to get awesome multi-user features like topics and likes
- as HTML files to a static website, which you can host yourself for free on many external providers like GitHub Pages, and remain in full control
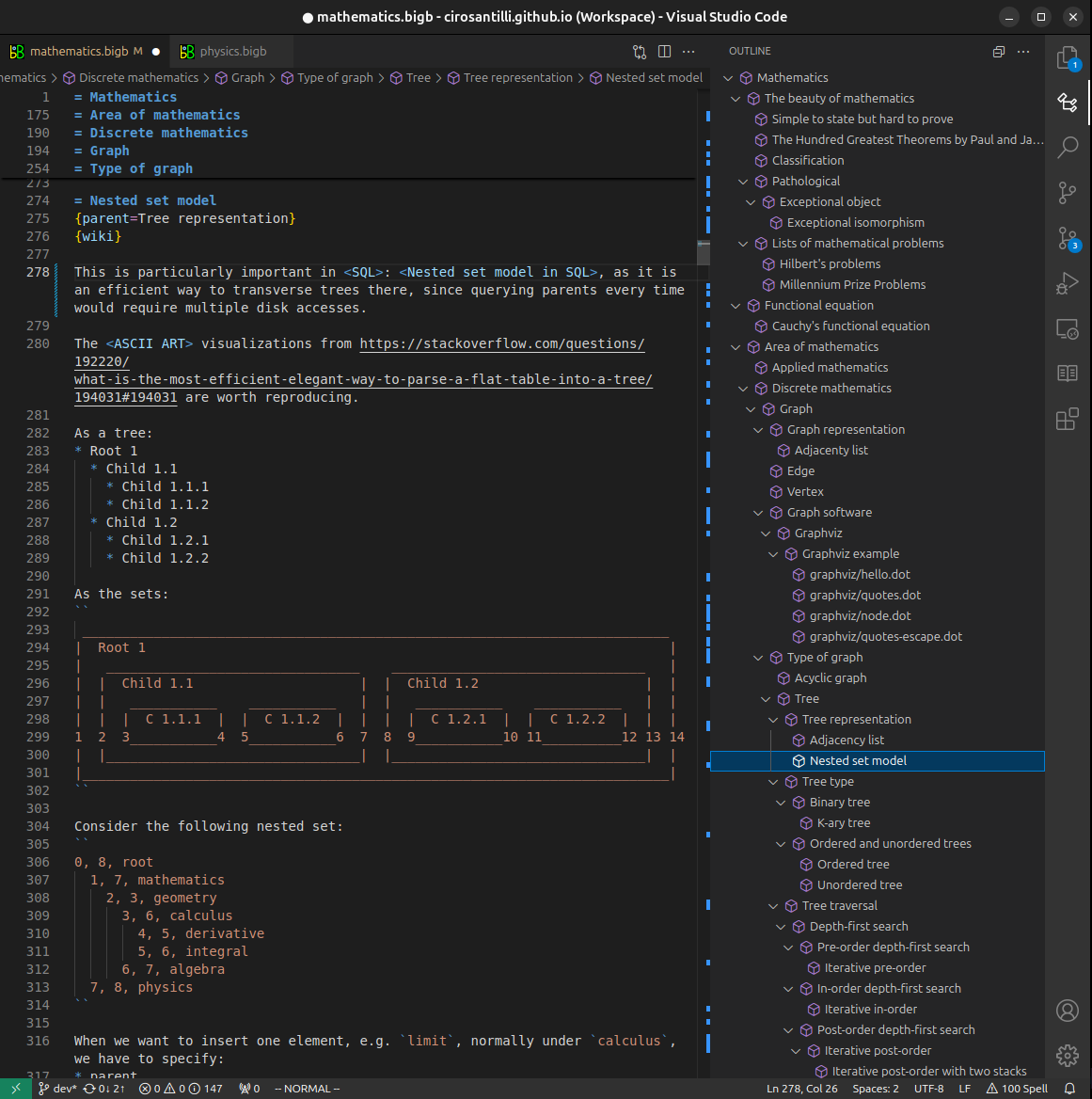
Figure 3. Visual Studio Code extension installation.Figure 4. Visual Studio Code extension tree navigation.Figure 5. Web editor. You can also edit articles on the Web editor without installing anything locally.Video 3. Edit locally and publish demo. Source. This shows editing OurBigBook Markup and publishing it using the Visual Studio Code extension.Video 4. OurBigBook Visual Studio Code extension editing and navigation demo. Source. - Infinitely deep tables of contents:
All our software is open source and hosted at: github.com/ourbigbook/ourbigbook
Further documentation can be found at: docs.ourbigbook.com
Feel free to reach our to us for any help or suggestions: docs.ourbigbook.com/#contact