The L2 Syntactic Complexity Analyzer is a tool designed to assess and analyze the syntactic complexity of written texts, particularly those produced by second language (L2) learners. It provides insights into the syntactic structures used in the writing, offering metrics that can indicate the proficiency level of the writer in terms of their ability to use complex sentences, varied sentence structures, and overall syntactic diversity.
The General Internet Corpus of Russian (Генеральный интернет-корпус русского языка) is a linguistic resource designed to represent the Russian language as it is used on the internet. Compiled from various online sources, this corpus includes texts from social media, blogs, forums, news sites, and other web-based platforms.
The Linguistic Data Consortium (LDC) is a non-profit organization based at the University of Pennsylvania that plays a crucial role in the field of linguistics and language resource development. Established in 1992, its primary mission is to facilitate the creation and distribution of linguistic data, resources, and annotations to support research and development in linguistics, natural language processing, speech recognition, and related areas.
Logology is a branch of linguistics that focuses on the study of words, particularly their formation, meanings, and structures. It often intersects with areas like morphology (the study of word structure) and lexicology (the study of the meaning and use of words). Logology can encompass various linguistic phenomena, including how new words are coined, how existing words evolve, and the relationships between different words within a language.
Error analysis is a subfield of applied linguistics that focuses on identifying, categorizing, and understanding the mistakes that language learners make when acquiring a new language. It involves studying the errors in learners' spoken or written language to gain insights into their learning processes, language acquisition stages, and the influence of their native language on the target language.
The International Association of Applied Linguistics (AILA) is a professional organization that promotes the study and application of linguistics in various real-world contexts. Founded in 1964, AILA aims to facilitate the exchange of knowledge and research among linguists, educators, and practitioners who apply linguistic principles in fields such as language education, language policy, translation, language assessment, and sociolinguistics. AILA organizes conferences, publishes journals, and encourages collaborations and networking among its members.
The International Corpus of English (ICE) is a large, systematic collection of English language data compiled from various regions around the world. It aims to provide a comprehensive resource for the study of English as it is used in different countries and contexts, focusing on both spoken and written forms of the language. ICE consists of several national components, each representing a specific variety of English, such as British, American, Australian, Canadian, Indian, and others.
Language delay refers to a situation in which a child does not achieve language development milestones at the expected age. It is characterized by a lag in the ability to understand or use language compared to peers. This can manifest in various ways, including: 1. **Expressive Language Delay**: Difficulty in expressing thoughts and ideas verbally. A child may have a limited vocabulary, struggle with grammar, or may not be forming sentences appropriately.
Speech-language pathology is a field of healthcare that focuses on the evaluation, diagnosis, and treatment of communication and swallowing disorders. Speech-language pathologists (SLPs), also known as speech therapists, work with individuals of all ages, from infants to the elderly, to address a variety of issues related to speech, language, voice, and fluency.
The Oxford English Corpus (OEC) is a large and continually updated linguistic database created by Oxford University Press. It contains a broad range of text samples from various sources, including books, newspapers, websites, and more, reflecting contemporary English usage across different genres, registers, and contexts. The corpus is designed to provide insights into the evolving nature of the English language, including the frequency and usage of words, phrases, and grammatical structures.
Quantitative linguistics is a subfield of linguistics that applies quantitative methods and statistical techniques to analyze linguistic data. The goal is to uncover patterns, trends, and relationships in language use across various dimensions, including phonetics, syntax, semantics, and sociolinguistics. Researchers in quantitative linguistics employ a variety of tools and methodologies, including: - **Statistical Analysis**: Using statistical tests to validate hypotheses about language phenomena.
Realia refers to real-life objects, materials, or resources that are used in the process of education and translation to provide context and enhance understanding. In translation studies, realia can include cultural references, names of local products, customs, or specific terms that are unique to a particular place or culture. When translating, it is important to consider how to convey these elements to the target audience in a way that maintains their cultural significance.
Second-language acquisition (SLA) is the process by which individuals learn a language other than their native language. This can occur in various contexts, such as formal education settings, immersion environments, or informal settings through interaction with speakers of the language. SLA encompasses not just the learning of vocabulary and grammar, but also the development of listening, speaking, reading, and writing skills in the second language (L2).
A Polynomial-time Approximation Scheme (PTAS) is a type of algorithmic framework used to find approximate solutions to optimization problems, particularly those that are NP-hard. The key characteristics of a PTAS are: 1. **Approximation Guarantee**: Given an optimization problem and a function \( \epsilon > 0 \), a PTAS provides a solution that is within a factor of \( (1 + \epsilon) \) of the optimal solution.
The Alpha Max Plus Beta Min algorithm is a decision-making framework used primarily in multi-criteria decision analysis (MCDA) and operations research. It is useful for evaluating alternatives when there are multiple conflicting criteria. The basic idea behind this algorithm is to establish a systematic way to score or rank options based on their performance across different criteria. ### Key Components: 1. **Criteria**: The algorithm considers multiple criteria (attributes) that are important for evaluating alternatives.
The washback effect, also known as backwash effect, refers to the impact that assessments or testing can have on teaching and learning practices. This concept highlights the idea that the way students are assessed can influence the methods teachers use in the classroom and the manner in which students learn. In positive terms, a strong alignmment between assessment and instructional goals can lead to effective teaching strategies that enhance learning.
The Wellington Corpus of Spoken New Zealand English is a linguistic resource that comprises a collection of spoken language data collected in various contexts from speakers of New Zealand English. Developed at Victoria University of Wellington, this corpus is designed to represent the everyday spoken language used in New Zealand, capturing various demographics, social settings, and speaking styles. The corpus typically includes recordings of spontaneous conversations, interviews, and other forms of interaction, allowing researchers to analyze language use in a naturalistic setting.
The \( k \)-hitting set problem is a well-known problem in combinatorial optimization and theoretical computer science.
Property testing is a fundamental concept in computer science and, more specifically, in the field of algorithms and complexity theory. It involves the following key ideas: 1. **Definition**: Property testing is the process of determining whether a given object (often a function, graph, or dataset) exhibits a certain property or is "far" from having that property, without needing to examine the entire object. It is a randomized algorithmic technique that allows for efficient checks.
Guillermo Vargas Aignasse is an Argentine politician known for his role within the political landscape of Argentina. He has served in various capacities, including as a member of the provincial legislature in Tucumán. His political career includes involvement in agricultural and social issues, as well as contributions to local governance.
Pinned article: Introduction to the OurBigBook Project
Welcome to the OurBigBook Project! Our goal is to create the perfect publishing platform for STEM subjects, and get university-level students to write the best free STEM tutorials ever.
Everyone is welcome to create an account and play with the site: ourbigbook.com/go/register. We belive that students themselves can write amazing tutorials, but teachers are welcome too. You can write about anything you want, it doesn't have to be STEM or even educational. Silly test content is very welcome and you won't be penalized in any way. Just keep it legal!
Intro to OurBigBook
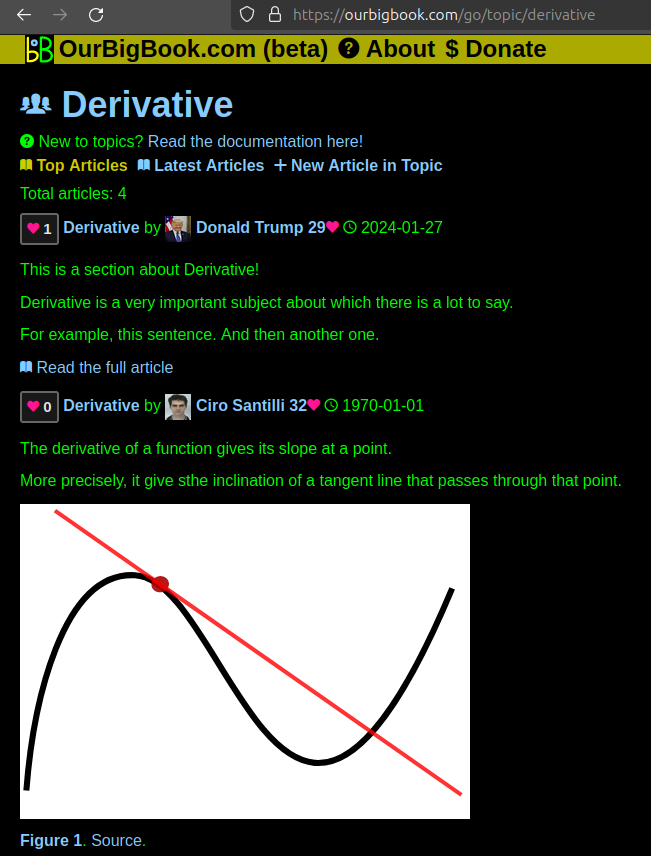
. Source. We have two killer features:
- topics: topics group articles by different users with the same title, e.g. here is the topic for the "Fundamental Theorem of Calculus" ourbigbook.com/go/topic/fundamental-theorem-of-calculusArticles of different users are sorted by upvote within each article page. This feature is a bit like:
- a Wikipedia where each user can have their own version of each article
- a Q&A website like Stack Overflow, where multiple people can give their views on a given topic, and the best ones are sorted by upvote. Except you don't need to wait for someone to ask first, and any topic goes, no matter how narrow or broad
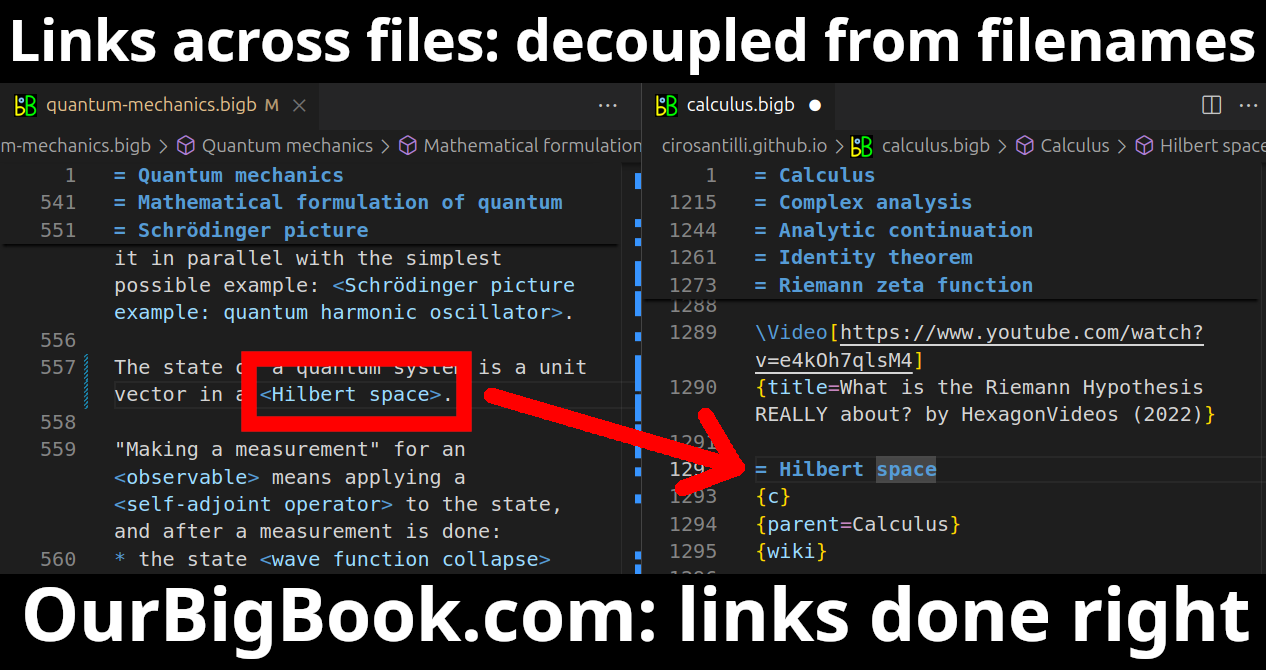
This feature makes it possible for readers to find better explanations of any topic created by other writers. And it allows writers to create an explanation in a place that readers might actually find it.Figure 1. Screenshot of the "Derivative" topic page. View it live at: ourbigbook.com/go/topic/derivativeVideo 2. OurBigBook Web topics demo. Source. - local editing: you can store all your personal knowledge base content locally in a plaintext markup format that can be edited locally and published either:This way you can be sure that even if OurBigBook.com were to go down one day (which we have no plans to do as it is quite cheap to host!), your content will still be perfectly readable as a static site.
- to OurBigBook.com to get awesome multi-user features like topics and likes
- as HTML files to a static website, which you can host yourself for free on many external providers like GitHub Pages, and remain in full control
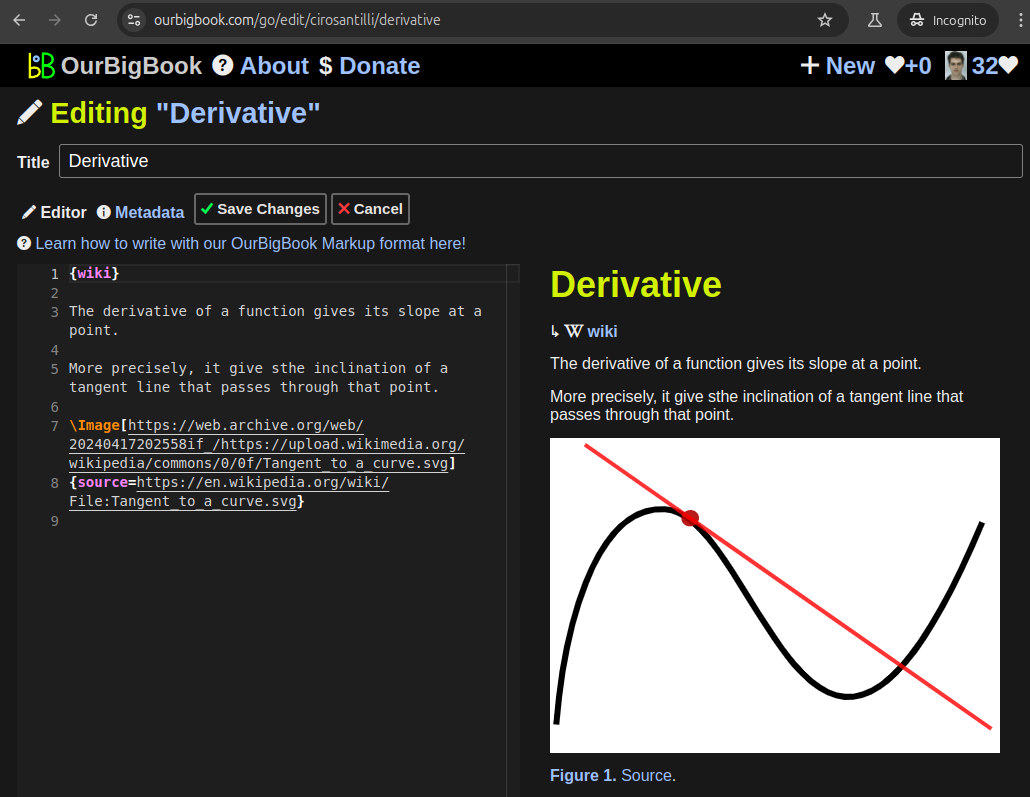
Figure 3. Visual Studio Code extension installation.Figure 4. Visual Studio Code extension tree navigation.Figure 5. Web editor. You can also edit articles on the Web editor without installing anything locally.Video 3. Edit locally and publish demo. Source. This shows editing OurBigBook Markup and publishing it using the Visual Studio Code extension.Video 4. OurBigBook Visual Studio Code extension editing and navigation demo. Source. - Infinitely deep tables of contents:
All our software is open source and hosted at: github.com/ourbigbook/ourbigbook
Further documentation can be found at: docs.ourbigbook.com
Feel free to reach our to us for any help or suggestions: docs.ourbigbook.com/#contact