Vocal music Created 2024-09-15 Updated 2025-07-16
Instrumental music Created 2024-09-15 Updated 2025-07-16
Jazz subgenre Created 2024-09-15 Updated 2025-07-16
Jazz artist Created 2024-09-15 Updated 2025-07-16
Jazz song Created 2024-09-15 Updated 2025-07-16
Jazz album Created 2024-09-15 Updated 2025-07-16
Category of chemical element Created 2024-09-15 Updated 2025-07-16
Discovery of chemical elements Created 2024-09-15 Updated 2025-07-16
Vocal and instrumental music Created 2024-09-15 Updated 2025-07-16
Requiem Created 2024-09-15 Updated 2025-07-16
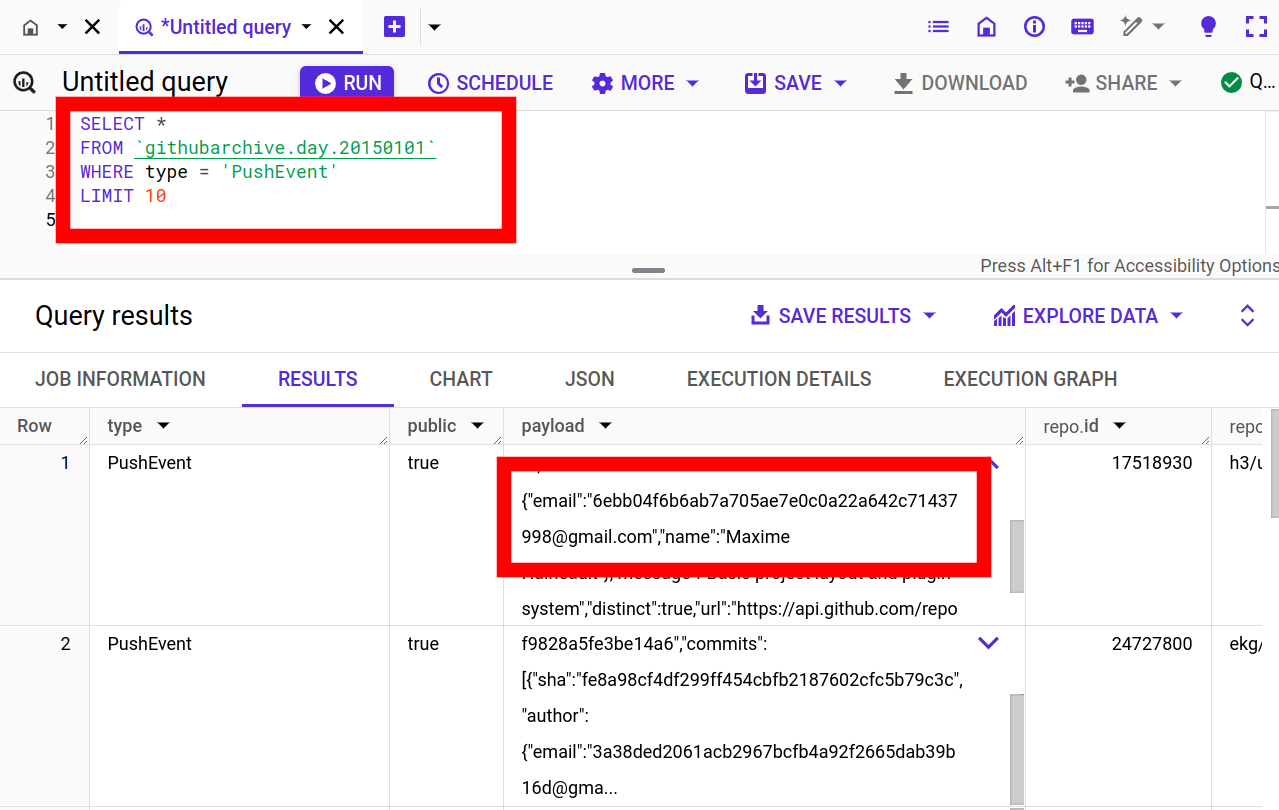
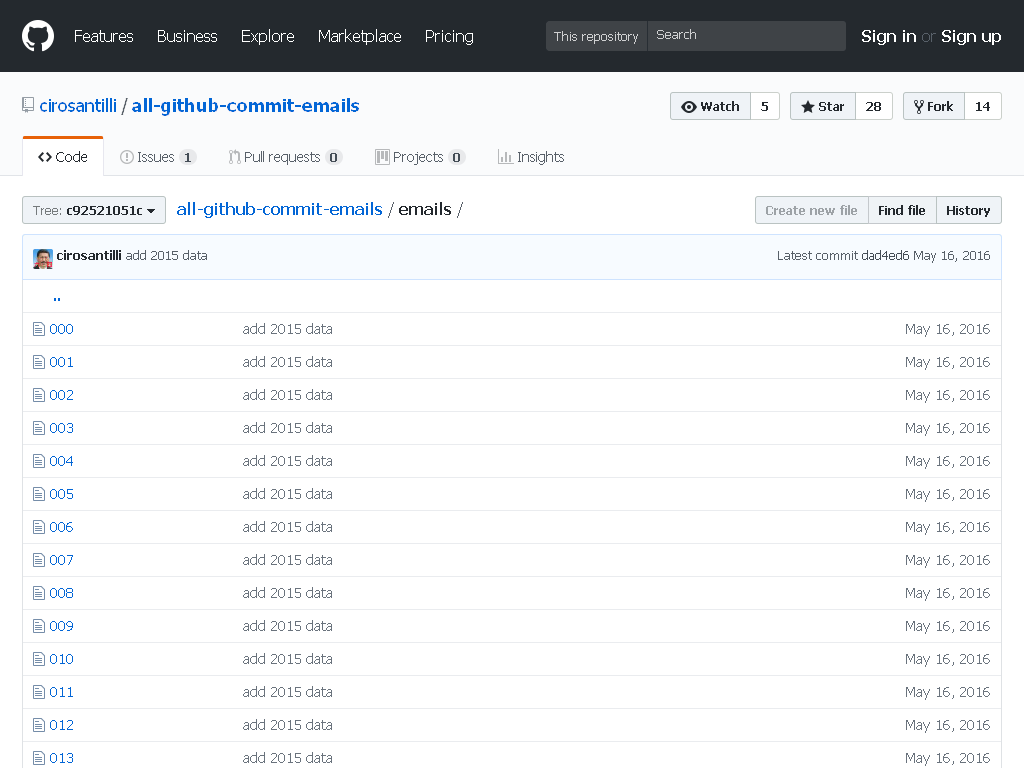
Aratu Week 2024 Talk by Ciro Santilli: My Best Random Projects All GitHub commit emails Created 2024-09-15 Updated 2025-07-16
Updates Relationship between the Falun Mine and the discovery of new chemical elements Created 2024-09-15 Updated 2025-07-16
- some cool chemical discoveries have been made with a relation to the mine, notably tantalum and selenium, added a section to Wikipedia: en.wikipedia.org/w/index.php?title=Falun_Mine&oldid=1245374294#Discovery_of_new_elements I used the book discovery Of The Elements by Mary Elvira Weeks as my primary reference.
- it is the Chinese version of the Scunthorpe problem due to a naming conflict with Falun Gong, a censored new religion that was banned in China
Announcements:
Updates Does copyright transfer of papers to publishers affect when the paper enters the public domain? Created 2024-09-15 Updated 2025-07-16
academia.stackexchange.com/questions/213576/do-copyright-transfer-of-papers-to-publishers-affect-when-the-paper-enters-the-p Do copyright transfer of papers to publishers affect when the paper enters the public domain since copyright belongs to a corporation and not persons?
Neurodiversity Created 2024-09-07 Updated 2025-07-16
Alec Guinness Created 2024-09-07 Updated 2025-07-16
This dude is the best.
Alec Guinness Interview on Parkinson Talk Show about Star Wars 1977
. Source. George Lucas just orally gave him away an extra 0.5% on top of his 2% of the revenue one day before the release. Then later on when asked for written proof, he lowered it to 0.25%.
What an interview. The way he carries himself. The way he speaks. It's so charming!!!
Hachette v. Internet Archive Created 2024-09-07 Updated 2025-07-16
On the Quantum Theory of Radiation Created 2024-09-06 Updated 2025-07-16
History of stimulated emission Created 2024-09-06 Updated 2025-07-16
First postulated by Einstein in 1917 on his paper Zur Quantentheorie der Strahlung" ("On the Quantum Theory of Radiation") as a more elegant way to rederive Planck's law as part of the Einstein coefficients framework.
At that time there was no other physical evidence supporting the existence of the concept except that it looked more elegant.
Bibliography:
HTTPS Created 2024-09-06 Updated 2025-07-16
Aratu Week IV Created 2024-09-06 Updated 2025-07-16
There are unlisted articles, also show them or only show them.